Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice
- PMID: 28588307
- PMCID: PMC5460172
- DOI: 10.1038/s41598-017-02557-8
Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice
Abstract
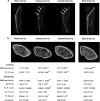
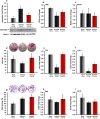
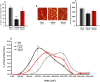
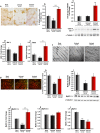
We previously showed that Irisin, a myokine released from skeletal muscle after physical exercise, plays a central role in the control of bone mass. Here we report that treatment with recombinant Irisin prevented bone loss in hind-limb suspended mice when administered during suspension (preventive protocol) and induced recovery of bone mass when mice were injected after bone loss due to a suspension period of 4 weeks (curative protocol). MicroCT analysis of femurs showed that r-Irisin preserved both cortical and trabecular bone mineral density, and prevented a dramatic decrease of the trabecular bone volume fraction. Moreover, r-Irisin protected against muscle mass decline in the hind-limb suspended mice, and maintained the fiber cross-sectional area. Notably, the decrease of myosin type II expression in unloaded mice was completely prevented by r-Irisin administration. Our data reveal for the first time that Irisin retrieves disuse-induced bone loss and muscle atrophy. These findings may lead to development of an Irisin-based therapy for elderly immobile osteoporotic and physically disable patients, and might represent a countermeasure for astronauts subjected to microgravity-induced bone and muscle losses.
Conflict of interest statement
M. Grano, S. Cinti, G. Colaianni, C. Cuscito, S. Colucci, G. Mori, G. Brunetti are name inventors of the Italian patent (MI2015A000558) and the European patent (16165324.1-1453) titled “Irisin for care and prevention of osteoporosis” related to the work described.
Figures



References
-
- Zehnder Y, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;15(3):180–9. doi: 10.1007/s00198-003-1529-6. - DOI - PubMed
-
- LeBlanc A, et al. Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Neuronal Interact. 2000;1(2):157–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

