Biological imaging using light-addressable potentiometric sensors and scanning photo-induced impedance microscopy
- PMID: 28588418
- PMCID: PMC5454363
- DOI: 10.1098/rspa.2017.0130
Biological imaging using light-addressable potentiometric sensors and scanning photo-induced impedance microscopy
Abstract
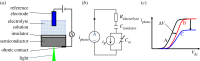
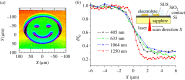
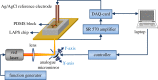
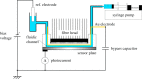
Light-addressable potentiometric sensors (LAPS) and scanning photo-induced impedance microscopy (SPIM) use photocurrent measurements at electrolyte-insulator-semiconductor substrates for spatio-temporal imaging of electrical potentials and impedance. The techniques have been used for the interrogation of sensor arrays and the imaging of biological systems. Sensor applications range from the detection of different types of ions and the label-free detection of charged molecules such as DNA and proteins to enzyme-based biosensors. Imaging applications include the temporal imaging of extracellular potentials and dynamic concentration changes in microfluidic channels and the lateral imaging of cell surface charges and cell metabolism. This paper will investigate the current state of the art of the measurement technology with a focus on spatial and temporal resolution and review the biological applications, these techniques have been used for. An outlook on future developments in the field will be given.
Keywords: biosensors; electrochemical imaging; impedance; ion concentrations; potentiometric sensor.
Conflict of interest statement
We do not have any competing interests.
Figures


Similar articles
-
Recent Developments of High-Resolution Chemical Imaging Systems Based on Light-Addressable Potentiometric Sensors (LAPSs).Sensors (Basel). 2019 Oct 3;19(19):4294. doi: 10.3390/s19194294. Sensors (Basel). 2019. PMID: 31623395 Free PMC article. Review.
-
LAPS and SPIM Imaging Using ITO-Coated Glass as the Substrate Material.Anal Chem. 2017 Aug 1;89(15):8129-8133. doi: 10.1021/acs.analchem.7b01898. Epub 2017 Jul 17. Anal Chem. 2017. PMID: 28678477
-
Light-Addressable Electrochemical Sensors toward Spatially Resolved Biosensing and Imaging Applications.ACS Sens. 2022 Jul 22;7(7):1791-1807. doi: 10.1021/acssensors.2c00940. Epub 2022 Jun 28. ACS Sens. 2022. PMID: 35762514 Review.
-
Scanning Electrochemical Photometric Sensors for Label-Free Single-Cell Imaging and Quantitative Absorption Analysis.Anal Chem. 2020 Jul 21;92(14):9739-9744. doi: 10.1021/acs.analchem.0c01118. Epub 2020 Jun 3. Anal Chem. 2020. PMID: 32437169
-
Light-Addressable Potentiometric Sensors Using ZnO Nanorods as the Sensor Substrate for Bioanalytical Applications.Anal Chem. 2018 Jul 17;90(14):8708-8715. doi: 10.1021/acs.analchem.8b02244. Epub 2018 Jul 6. Anal Chem. 2018. PMID: 29932632
Cited by
-
InGaN as a Substrate for AC Photoelectrochemical Imaging.Sensors (Basel). 2019 Oct 11;19(20):4386. doi: 10.3390/s19204386. Sensors (Basel). 2019. PMID: 31614420 Free PMC article.
-
Recent Developments of High-Resolution Chemical Imaging Systems Based on Light-Addressable Potentiometric Sensors (LAPSs).Sensors (Basel). 2019 Oct 3;19(19):4294. doi: 10.3390/s19194294. Sensors (Basel). 2019. PMID: 31623395 Free PMC article. Review.
-
High Spatial Resolution Ion Imaging by Focused Electron-Beam Excitation with Nanometric Thin Sensor Substrate.Sensors (Basel). 2022 Feb 1;22(3):1112. doi: 10.3390/s22031112. Sensors (Basel). 2022. PMID: 35161857 Free PMC article.
References
-
- Nakao M, Yoshinobu T, Iwasaki H. 1994. Scanning-laser-beam semiconductor pH-imaging sensor. Sens. Actuat. B Chem. 20, 119–123. (doi:10.1016/0925-4005(93)01199-E) - DOI
-
- Werner CF, et al. 2011. Determination of the extracellular acidification of Escherichia coli by a light-addressable potentiometric sensor. Phys. Status Solidi A 208, 1340–1344. (doi:10.1002/pssa.201001141) - DOI
-
- Wu C, Poghossian A, Bronder TS, Schöning MJ. 2016. Sensing of double-stranded DNA molecules by their intrinsic molecular charge using the light-addressable potentiometric sensor. Sens. Actuat. B Chem. 229, 506–512. (doi:10.1016/j.snb.2016.02.004) - DOI
-
- Zhang D-W, Wu F, Wang J, Watkinson M, Krause S. 2016. Image detection of yeast Saccharomyces cerevisiae by light-addressable potentiometric sensors (LAPS). Electrochem. Commun. 72, 41–45. (doi:10.1016/j.elecom.2016.08.017) - DOI
-
- Stein B, George M, Gaub HE, Parak WJ. 2004. Extracellular measurements of averaged ionic currents with the light-addressable potentiometric sensor (LAPS). Sens. Actuat. B Chem. 98, 299–304. (doi:10.1016/j.snb.2003.10.034) - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

