Long-Term Assessment of AAV-Mediated Zinc Finger Nuclease Expression in the Mouse Brain
- PMID: 28588449
- PMCID: PMC5440507
- DOI: 10.3389/fnmol.2017.00142
Long-Term Assessment of AAV-Mediated Zinc Finger Nuclease Expression in the Mouse Brain
Abstract
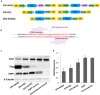
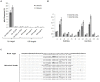
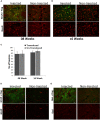
Gene editing tools like TALENs, ZFNs and Crispr/Cas now offer unprecedented opportunities for targeted genetic manipulations in virtually all species. Most of the recent research in this area has concentrated on manipulation of the genome in isolated cells, which then give rise to transgenic animals or modified stem cell lines. Much less is known about applicability of genetic scissors in terminally differentiated, non-dividing cells like neurons of the adult brain. We addressed this question by expression of a pair of ZFNs targeting the murine cathepsin D gene in CNS neurons by means of an optimized AAV viral vector. We show that ZFN expression resulted in substantial depletion of cathepsin D from neuronal lysosomes, demonstrating a robust gene deletion. Importantly, long-term ZFN expression in CNS neurons did not impair essential neuronal functionality and did not cause inflammation or neurodegeneration, suggesting that potent genetic scissors can be expressed safely in the mouse brain. This finding opens up new venues to create novel research models for neurodegenerative disorders.
Keywords: adeno-associated virus; cathepsin D; genome editing; in vivo; zinc finger nuclease.
Figures



Similar articles
-
Codon swapping of zinc finger nucleases confers expression in primary cells and in vivo from a single lentiviral vector.Curr Gene Ther. 2014;14(5):365-76. doi: 10.2174/156652321405140926161748. Curr Gene Ther. 2014. PMID: 25687502
-
Temperature effect on CRISPR-Cas9 mediated genome editing.J Genet Genomics. 2017 Apr 20;44(4):199-205. doi: 10.1016/j.jgg.2017.03.004. Epub 2017 Mar 30. J Genet Genomics. 2017. PMID: 28412228
-
Non-viral Delivery of Zinc Finger Nuclease mRNA Enables Highly Efficient In Vivo Genome Editing of Multiple Therapeutic Gene Targets.Mol Ther. 2019 Apr 10;27(4):866-877. doi: 10.1016/j.ymthe.2019.03.003. Epub 2019 Mar 7. Mol Ther. 2019. PMID: 30902585 Free PMC article.
-
Basics of genome editing technology and its application in livestock species.Reprod Domest Anim. 2017 Aug;52 Suppl 3:4-13. doi: 10.1111/rda.13012. Reprod Domest Anim. 2017. PMID: 28815851 Review.
-
Origins of Programmable Nucleases for Genome Engineering.J Mol Biol. 2016 Feb 27;428(5 Pt B):963-89. doi: 10.1016/j.jmb.2015.10.014. Epub 2015 Oct 23. J Mol Biol. 2016. PMID: 26506267 Free PMC article. Review.
Cited by
-
Nanoparticle-mediated delivery of non-viral gene editing technology to the brain.Prog Neurobiol. 2024 Jan;232:102547. doi: 10.1016/j.pneurobio.2023.102547. Epub 2023 Dec 1. Prog Neurobiol. 2024. PMID: 38042249 Free PMC article. Review.
-
Long-lasting analgesia via targeted in situ repression of NaV1.7 in mice.Sci Transl Med. 2021 Mar 10;13(584):eaay9056. doi: 10.1126/scitranslmed.aay9056. Sci Transl Med. 2021. PMID: 33692134 Free PMC article.
-
Homogenous generation of dopaminergic neurons from multiple hiPSC lines by transient expression of transcription factors.Cell Death Dis. 2019 Nov 27;10(12):898. doi: 10.1038/s41419-019-2133-9. Cell Death Dis. 2019. PMID: 31776327 Free PMC article.
-
HIV Eradication Strategies: Implications for the Central Nervous System.Curr HIV/AIDS Rep. 2019 Feb;16(1):96-104. doi: 10.1007/s11904-019-00428-7. Curr HIV/AIDS Rep. 2019. PMID: 30734905 Free PMC article. Review.
-
Gene therapy for CNS disorders: modalities, delivery and translational challenges.Nat Rev Neurosci. 2024 Aug;25(8):553-572. doi: 10.1038/s41583-024-00829-7. Epub 2024 Jun 19. Nat Rev Neurosci. 2024. PMID: 38898231 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

