Bacterial Biogeography across the Amazon River-Ocean Continuum
- PMID: 28588561
- PMCID: PMC5440517
- DOI: 10.3389/fmicb.2017.00882
Bacterial Biogeography across the Amazon River-Ocean Continuum
Abstract
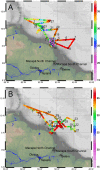
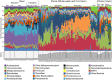
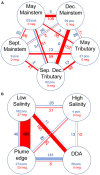
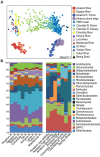
Spatial and temporal patterns in microbial biodiversity across the Amazon river-ocean continuum were investigated along ∼675 km of the lower Amazon River mainstem, in the Tapajós River tributary, and in the plume and coastal ocean during low and high river discharge using amplicon sequencing of 16S rRNA genes in whole water and size-fractionated samples (0.2-2.0 μm and >2.0 μm). River communities varied among tributaries, but mainstem communities were spatially homogeneous and tracked seasonal changes in river discharge and co-varying factors. Co-occurrence network analysis identified strongly interconnected river assemblages during high (May) and low (December) discharge periods, and weakly interconnected transitional assemblages in September, suggesting that this system supports two seasonal microbial communities linked to river discharge. In contrast, plume communities showed little seasonal differences and instead varied spatially tracking salinity. However, salinity explained only a small fraction of community variability, and plume communities in blooms of diatom-diazotroph assemblages were strikingly different than those in other high salinity plume samples. This suggests that while salinity physically structures plumes through buoyancy and mixing, the composition of plume-specific communities is controlled by other factors including nutrients, phytoplankton community composition, and dissolved organic matter chemistry. Co-occurrence networks identified interconnected assemblages associated with the highly productive low salinity near-shore region, diatom-diazotroph blooms, and the plume edge region, and weakly interconnected assemblages in high salinity regions. This suggests that the plume supports a transitional community influenced by immigration of ocean bacteria from the plume edge, and by species sorting as these communities adapt to local environmental conditions. Few studies have explored patterns of microbial diversity in tropical rivers and coastal oceans. Comparison of Amazon continuum microbial communities to those from temperate and arctic systems suggest that river discharge and salinity are master variables structuring a range of environmental conditions that control bacterial communities across the river-ocean continuum.
Keywords: Amazon River; Columbia River; diatom-diazotroph assemblage; freshwater bacteria; marine bacteria; microbial diversity; river plume; tropical Atlantic Ocean.
Figures

References
-
- Alonso-Saez L., Aristegui J., Pinhassi J., Gomez-Consarnau L., Gonzalez J. M., Vaque D., et al. (2007). Bacterial assemblage structure and carbon metabolism along a productivity gradient in the NE Atlantic Ocean. Aquat. Microb. Ecol. 46 43–53. 10.3354/ame046043 - DOI
-
- Amend A. S., Oliver T. A., Amaral-Zettler L. A., Boetius A., Fuhrman J. A., Horner-Devine M. C., et al. (2013). Macroecological patterns of marine bacteria on a global scale. J. Biogeogr. 40 800–811. 10.1111/jbi.12034 - DOI
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. Ser. B-Methodol. 57 289–300.
-
- Benner R., Opsahl S., ChinLeo G., Richey J. E., Forsberg B. R. (1995). Bacterial carbon metabolism in the Amazon River system. Limnol. Oceanogr. 40 1262–1270. 10.4319/lo.1995.40.7.1262 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

