Biofilm vs. Planktonic Lifestyle: Consequences for Pesticide 2,4-D Metabolism by Cupriavidus necator JMP134
- PMID: 28588567
- PMCID: PMC5440565
- DOI: 10.3389/fmicb.2017.00904
Biofilm vs. Planktonic Lifestyle: Consequences for Pesticide 2,4-D Metabolism by Cupriavidus necator JMP134
Abstract
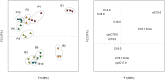
The development of bacterial biofilms in natural environments may alter important functions, such as pollutant bioremediation by modifying both the degraders' physiology and/or interactions within the matrix. The present study focuses on the influence of biofilm formation on the metabolism of a pesticide, 2,4-dichlorophenoxyacetic acid (2,4-D), by Cupriavidus necator JMP134. Pure cultures were established in a liquid medium with 2,4-D as a sole carbon source with or without sand grains for 10 days. Bacterial numbers and 2,4-D concentrations in solution were followed by spectrophotometry, the respiration rate by gas chromatography and the surface colonization by electron microscopy. In addition, isotopic techniques coupled with Fatty Acid Methyl Ester (FAME) profiling were used to determine possible metabolic changes. After only 3 days, approximately 80% of the cells were attached to the sand grains and microscopy images showed that the porous medium was totally clogged by the development of a biofilm. After 10 days, there was 25% less 2,4-D in the solution in samples with sand than in control samples. This difference was due to (1) a higher (+8%) mineralization of 2,4-D by sessile bacteria and (2) a retention (15%) of 2,4-D in the biofilm matrix. Besides, the amount of carbohydrates, presumably constituting the biofilm polysaccharides, increased by 63%. Compound-specific isotope analysis revealed that the FAME isotopic signature was less affected by the biofilm lifestyle than was the FAME composition. These results suggest that sessile bacteria differ more in their anabolism than in their catabolism compared to their planktonic counterparts. This study stresses the importance of considering interactions between microorganisms and their habitat when studying pollutant dynamics in porous media.
Keywords: 2,4-D; Cupriavidus necator JMP134; PLFA-SIP; biofilm.
Figures




References
-
- Baath E., Anderson T. H. (2003). Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 35, 955–963. 10.1016/S0038-0717(03)00154-8 - DOI
-
- Boschker H. T. S., Nold S. C., Wellsbury P., Bos D., de Graaf W., Pel R., et al. (1998). Direct linking of microbial populations to specific biogeochemical processes by C-13-labelling of biomarkers. Nature 392, 801–805. 10.1038/33900 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

