E-cadherin: A potential biomarker of colorectal cancer prognosis
- PMID: 28588719
- PMCID: PMC5452924
- DOI: 10.3892/ol.2017.6063
E-cadherin: A potential biomarker of colorectal cancer prognosis
Abstract
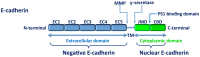
Colorectal cancer (CRC) is a common and lethal disease. It is the third most common type of cancer in the world, behind lung and breast cancer, with almost 1.4 million new cases diagnosed in 2012. The risk of developing CRC is influenced by environmental and genetic factors. Adenocarcinomas comprise the vast majority (98%) of CRCs. A patient's likelihood of survival is associated with the tumor stage at the time of diagnosis. With regular screening, CRC can be identified early, when treatment is the most effective. However, CRC is typically asymptomatic until the advanced stages. The combination of the absence of symptoms and current screening methodology results in a significant number of patients being diagnosed in advanced stages. The purpose of the present review is to discuss and summarize the biomarkers linked to CRC progression, particularly the controversial E-cadherin, which is a calcium-dependent cell-cell adhesion molecule involved in the mesenchymal-epithelial transition.
Keywords: E-cadherin protein; biomarkers; colorectal cancer.
Figures


Similar articles
-
Elevated soluble E-cadherin during the epithelial-mesenchymal transition process and as a diagnostic marker in colorectal cancer.Gene. 2020 Sep 5;754:144899. doi: 10.1016/j.gene.2020.144899. Epub 2020 Jun 13. Gene. 2020. PMID: 32544494
-
N-cadherin, a novel prognostic biomarker, drives malignant progression of colorectal cancer.Mol Med Rep. 2015 Aug;12(2):2999-3006. doi: 10.3892/mmr.2015.3687. Epub 2015 Apr 27. Mol Med Rep. 2015. PMID: 25936636
-
Syndecan-1, Epithelial-Mesenchymal Transition Markers (E-cadherin/β-catenin) and Neoangiogenesis-related Proteins (PCAM-1 and Endoglin) in Colorectal Cancer.Anticancer Res. 2016 May;36(5):2271-80. Anticancer Res. 2016. PMID: 27127133
-
New trends in molecular and cellular biomarker discovery for colorectal cancer.World J Gastroenterol. 2016 Jul 7;22(25):5678-93. doi: 10.3748/wjg.v22.i25.5678. World J Gastroenterol. 2016. PMID: 27433083 Free PMC article. Review.
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
Cited by
-
Electrochemical Biosensors for Determination of Colorectal Tumor Biomarkers.Micromachines (Basel). 2020 Apr 14;11(4):411. doi: 10.3390/mi11040411. Micromachines (Basel). 2020. PMID: 32295170 Free PMC article. Review.
-
miR614 Expression Enhances Breast Cancer Cell Motility.Int J Mol Sci. 2020 Dec 24;22(1):112. doi: 10.3390/ijms22010112. Int J Mol Sci. 2020. PMID: 33374314 Free PMC article.
-
Investigation of the expression levels of CDH1, FHIT, PTEN, and TTPAL genes in colorectal tumors.Turk J Med Sci. 2022 Feb;52(1):124-130. doi: 10.3906/sag-2110-296. Epub 2022 Feb 22. Turk J Med Sci. 2022. PMID: 36161592 Free PMC article.
-
Use of STAT6 Phosphorylation Inhibitor and Trimethylglycine as New Adjuvant Therapies for 5-Fluorouracil in Colitis-Associated Tumorigenesis.Int J Mol Sci. 2020 Mar 20;21(6):2130. doi: 10.3390/ijms21062130. Int J Mol Sci. 2020. PMID: 32244885 Free PMC article.
-
Integrating E-cadherin expression levels with TNM staging for enhanced prognostic prediction in colorectal cancer patients.BMC Cancer. 2025 Jan 27;25(1):150. doi: 10.1186/s12885-025-13539-9. BMC Cancer. 2025. PMID: 39871234 Free PMC article.
References
-
- Institut National Du Cancer (INCA), corp-author Les traitements du cancer du côlon, collection Guides patients Cancer info. http://www.e-cancer.fr/Patients-et-proches/Les-cancers/Cancer-du-colon/P... INCA. 2010
LinkOut - more resources
Full Text Sources
Other Literature Sources
