Palliative treatment of patients with inoperable locally advanced, recurrent or metastatic head and neck squamous cell cancer, using a low-dose and personalized chemotherapeutic regimen
- PMID: 28588721
- PMCID: PMC5452905
- DOI: 10.3892/ol.2017.6068
Palliative treatment of patients with inoperable locally advanced, recurrent or metastatic head and neck squamous cell cancer, using a low-dose and personalized chemotherapeutic regimen
Abstract
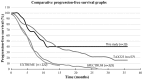
Inoperable or metastatic head and neck squamous cell cancer (HNSCC) is known to be associated with a poor patient prognosis. First line therapies include a Taxol, platinum-based antineoplastic and fluorouracil (FU) treatment regimen (TPF) or a platinum-based antineoplastic, FU and EGFR inhibitor treatment regimen (PFE). The toxicity of these regimens is one of the major limiting factors, particularly for palliative treatment. The present study is a retrospective study of 15 patients with HNSCC, where the treatment goal was palliative. Of the 15 patients, 8 received a TPF, while 7 received a PFE. A total of 129 treatment cycles were administered with a median of 9 cycles (range, 3-14). Chemotherapy began with low doses and was subsequently titrated up based on tolerance and response. Positive responses were noted with the lower doses compared with the conventional doses, and maximal doses were not required. The median dose of cisplatin, paclitaxel and 5-FU administered was 40 mg/m2, 80 mg/m2 and 360 mg/m2/day for 5 days, respectively. Cetuximab was used at a standard dose. At the initial follow-up (mean, 64 days; 3 cycles), a 100% disease control rate (DCR) and 80% overall response rate (ORR) was achieved. A positive response, 60% DCR and 60% ORR, was maintained until the late stages of the study (mean, 217 days; 9 cycles). Following termination of chemotherapy after >9 cycles, 4 patients remained disease free for ~1 year. A total of 3 patients exhibited a pathologic complete response despite radiologically exhibiting residual disease. The median progression-free survival time was 10.03 months and the overall survival time was 15.77 months. The only grade 3 hematologic toxicity noted was neutropenia in 3 (20%) patients. Grade 3 vomiting was noted in 1 (6.67%) patient and grade 3 stomatitis was noted in 1 (6.67%) patient. Due to low toxicity patients exhibited improved tolerance to this approach, particularly in terms of palliative care. Furthermore, these results are in contrast to the axiom that increased doses are more effective.
Keywords: low-dose chemotherapy; palliative chemotherapy; patient customized chemotherapy; recurrent HNSCC.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
