Maternal embryonic leucine zipper kinase inhibits epithelial-mesenchymal transition by regulating transforming growth factor-β signaling
- PMID: 28588728
- PMCID: PMC5452933
- DOI: 10.3892/ol.2017.6081
Maternal embryonic leucine zipper kinase inhibits epithelial-mesenchymal transition by regulating transforming growth factor-β signaling
Abstract
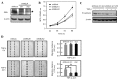
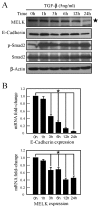
Maternal embryonic leucine zipper kinase (MELK) performs an important role in self-renewal and proliferation of progenitor cells or tumor stem cells, and is expressed in aggressive cancers, contributing to tumorigenesis. However, the function of MELK in metastasis is unknown. In the present study, the lung cancer A549 cell line was utilized in order to study the role of MELK in epithelial-mesenchymal transitions (EMTs), the initial step of tumor metastasis. It was identified that transforming growth factor-β (TGF-β) could downregulate the expression of MELK, and that MELK could inhibit EMT by regulating TGF-β signaling. MELK can interact with Smad proteins, which represses TGF-β/Smad-mediated signaling activity. The findings of the present study identified the effect of MELK in TGF-β signaling and the EMT process.
Keywords: A549; Smad; epithelial-mesenchymal transition; maternal embryonic leucine zipper kinase; transforming growth factor-β.
Figures
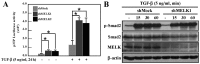

Similar articles
-
Maternal Embryonic Leucine Zipper Kinase is Associated with Metastasis in Triple-negative Breast Cancer.Cancer Res Commun. 2023 Jun 20;3(6):1078-1092. doi: 10.1158/2767-9764.CRC-22-0330. eCollection 2023 Jun. Cancer Res Commun. 2023. PMID: 37377604 Free PMC article.
-
Maternal embryonic leucine zipper kinase serves as a poor prognosis marker and therapeutic target in gastric cancer.Oncotarget. 2016 Feb 2;7(5):6266-80. doi: 10.18632/oncotarget.6673. Oncotarget. 2016. PMID: 26701722 Free PMC article.
-
Chinese medicine Bu-Fei decoction attenuates epithelial-mesenchymal transition of non-small cell lung cancer via inhibition of transforming growth factor β1 signaling pathway in vitro and in vivo.J Ethnopharmacol. 2017 May 23;204:45-57. doi: 10.1016/j.jep.2017.04.008. Epub 2017 Apr 12. J Ethnopharmacol. 2017. PMID: 28412214
-
Maternal embryonic leucine zipper kinase in tumor cells and tumor microenvironment: An emerging player and promising therapeutic opportunity.Cancer Lett. 2023 Apr 28;560:216126. doi: 10.1016/j.canlet.2023.216126. Epub 2023 Mar 16. Cancer Lett. 2023. PMID: 36933780 Review.
-
MELK: a potential novel therapeutic target for TNBC and other aggressive malignancies.Expert Opin Ther Targets. 2017 Sep;21(9):849-859. doi: 10.1080/14728222.2017.1363183. Epub 2017 Aug 16. Expert Opin Ther Targets. 2017. PMID: 28764577 Review.
Cited by
-
Anti-cancer immunotherapy using cancer-derived multiple epitope-peptides cocktail vaccination clinical studies in patients with refractory/persistent disease of uterine cervical cancer and ovarian cancer [phase 2].Oncoimmunology. 2020 Nov 11;9(1):1838189. doi: 10.1080/2162402X.2020.1838189. Oncoimmunology. 2020. PMID: 33235818 Free PMC article. Clinical Trial.
-
Maternal Embryonic Leucine Zipper Kinase Promotes Tumor Growth and Metastasis via Stimulating FOXM1 Signaling in Esophageal Squamous Cell Carcinoma.Front Oncol. 2020 Jan 28;10:10. doi: 10.3389/fonc.2020.00010. eCollection 2020. Front Oncol. 2020. PMID: 32047721 Free PMC article.
References
-
- Nakano I, Masterman-Smith M, Saigusa K, Paucar AA, Horvath S, Shoemaker L, Watanabe M, Negro A, Bajpai R, Howes A, et al. Maternal embryonic leucine zipper kinase is a key regulator of the proliferation of malignant brain tumors, including brain tumor stem cells. J Neurosci Res. 2008;86:48–60. doi: 10.1002/jnr.21471. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
