Conversion to monotherapy with luteinizing-hormone releasing hormone agonist or orchiectomy after reaching PSA nadir following maximal androgen blockade is able to prolong progression-free survival in patients with metastatic prostate cancer: A propensity score matching analysis
- PMID: 28588730
- PMCID: PMC5452888
- DOI: 10.3892/ol.2017.6056
Conversion to monotherapy with luteinizing-hormone releasing hormone agonist or orchiectomy after reaching PSA nadir following maximal androgen blockade is able to prolong progression-free survival in patients with metastatic prostate cancer: A propensity score matching analysis
Abstract
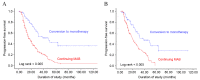
The present study evaluated androgen deprivation methods to determine the approach that most improves the progression-free survival (PFS) of patients with metastatic prostate cancer. Patients had received continuous maximal androgen blockade (MAB) or monotherapy [luteinizing-hormone releasing hormone (LHRH) agonist or orchiectomy] following the reaching of the prostate specific antigen (PSA) nadir. The medical records of 293 patients who received MAB following a diagnosis of metastatic prostate cancer were retrospectively reviewed. Following attainment of the PSA nadir and treatment with MAB, patients were maintained on continuous MAB (group CMAB) or converted to monotherapy (group MONO). Disease progression, defined as progression to castration-resistant prostate cancer, was evaluated and compared between the treatment modalities. PFS was compared between patients who received CMAB vs. MONO using 2:1 (102:53) propensity score matching; the basic clinicopathological characteristics (age, Gleason score, PSA and extent of bone metastasis) were similar between the groups. Disease progression was observed in 70.9% of all patients, with a median treatment period of 22.7 months. The median PFS time was 19.5 months in the CMAB group and 28.8 months in the MONO group (P=0.008). Kaplan-Meier analysis demonstrated that PFS was significantly associated with the type of maintenance androgen deprivation therapy (ADT; log rank <0.005). Multivariate analysis revealed that the type of maintenance ADT and the pretreatment extent of bone metastasis were independent predictors of prolonged PFS. In this propensity score matched-analysis, conversion to monotherapy with a LHRH agonist or orchiectomy following attainment of the PSA nadir with initial MAB, prolonged the PFS, suggesting that monotherapy maintenance following initial MAB may benefit patients by reducing side effects without decreasing treatment efficacy.
Keywords: androgen; disease-free survival; neoplasm metastasis; prostatic neoplasm.
Figures
Similar articles
-
[Predictive factor analysis of time to progression of castration-resistant prostate cancer after androgen deprivation therapy].Beijing Da Xue Xue Bao Yi Xue Ban. 2017 Aug 18;49(4):657-662. Beijing Da Xue Xue Bao Yi Xue Ban. 2017. PMID: 28816284 Chinese.
-
Survival outcomes of Chinese metastatic prostate cancer patients following primary androgen deprivation therapy in relation to prostate-specific antigen nadir level.Asia Pac J Clin Oncol. 2017 Apr;13(2):e65-e71. doi: 10.1111/ajco.12313. Epub 2014 Dec 3. Asia Pac J Clin Oncol. 2017. PMID: 25471685
-
Undetectable level of prostate specific antigen (PSA) nadir predicts PSA biochemical failure in local prostate cancer with delayed-combined androgen blockade.Jpn J Clin Oncol. 2008 Sep;38(9):617-22. doi: 10.1093/jjco/hyn071. Epub 2008 Aug 12. Jpn J Clin Oncol. 2008. PMID: 18697759 Clinical Trial.
-
[Maximal androgen blockade].Urologe A. 2000 Jan;39(1):27-35. doi: 10.1007/s001200050006. Urologe A. 2000. PMID: 10663193 Review. German.
-
EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer.Eur Urol. 2011 Apr;59(4):572-83. doi: 10.1016/j.eururo.2011.01.025. Epub 2011 Jan 25. Eur Urol. 2011. PMID: 21315502
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous