ZAR1 is a novel epigenetically inactivated tumour suppressor in lung cancer
- PMID: 28588743
- PMCID: PMC5457737
- DOI: 10.1186/s13148-017-0360-4
ZAR1 is a novel epigenetically inactivated tumour suppressor in lung cancer
Abstract
Background: Lung cancer is the leading cause of cancer-related deaths with 1.8 million new cases each year and poor 5-year prognosis. Promoter hypermethylation of tumour suppressors leads to their inactivation and thereby can promote cancer development and progression.
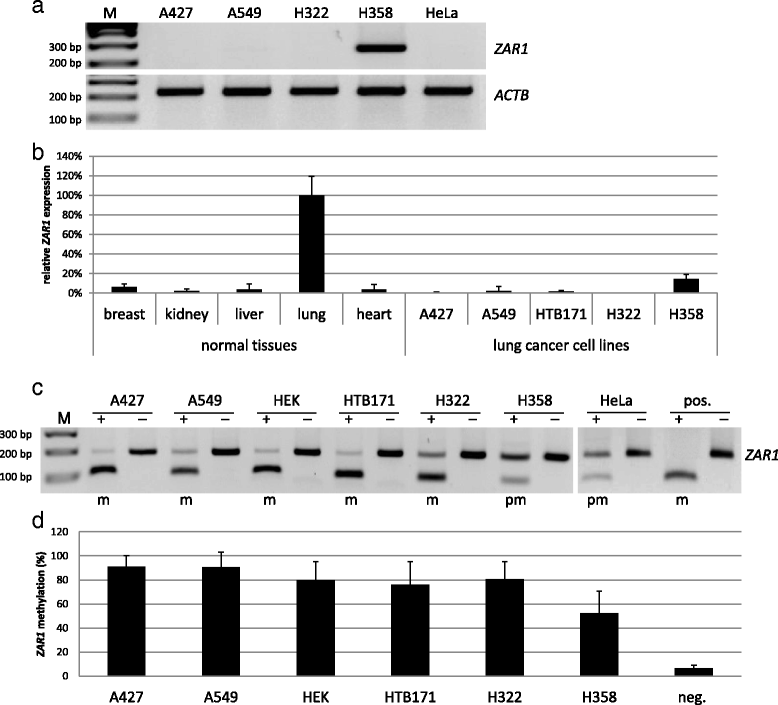
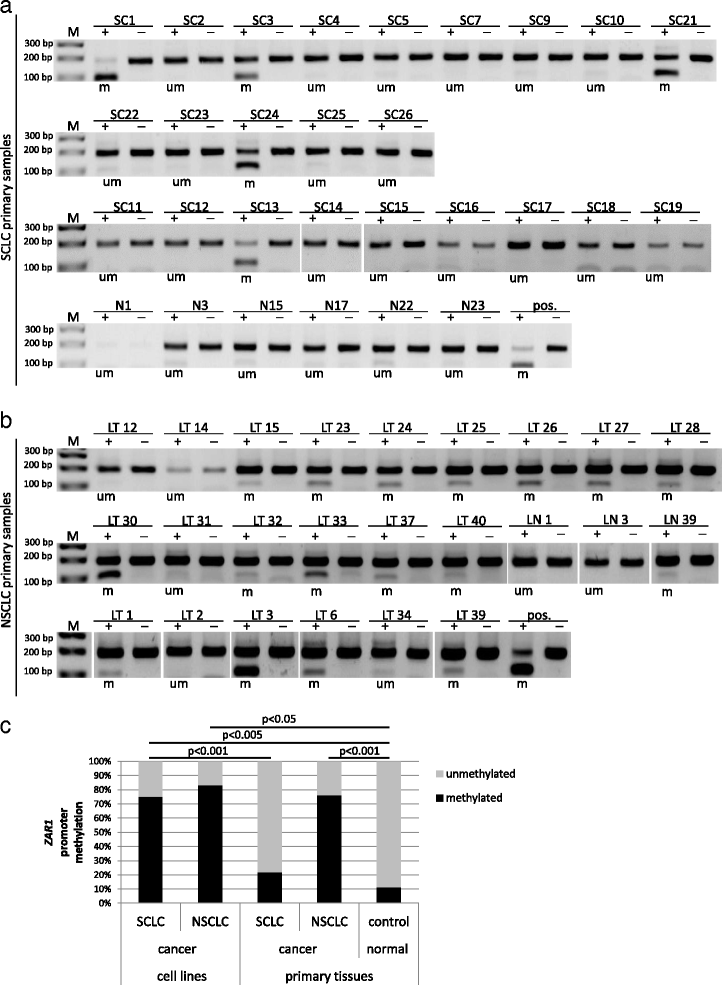
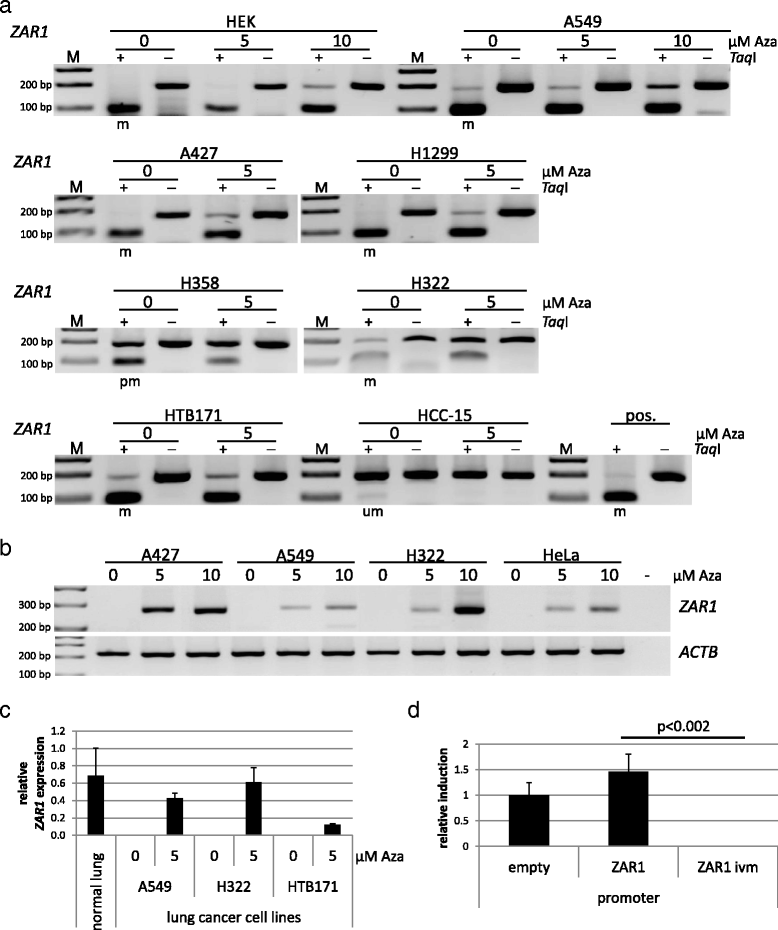
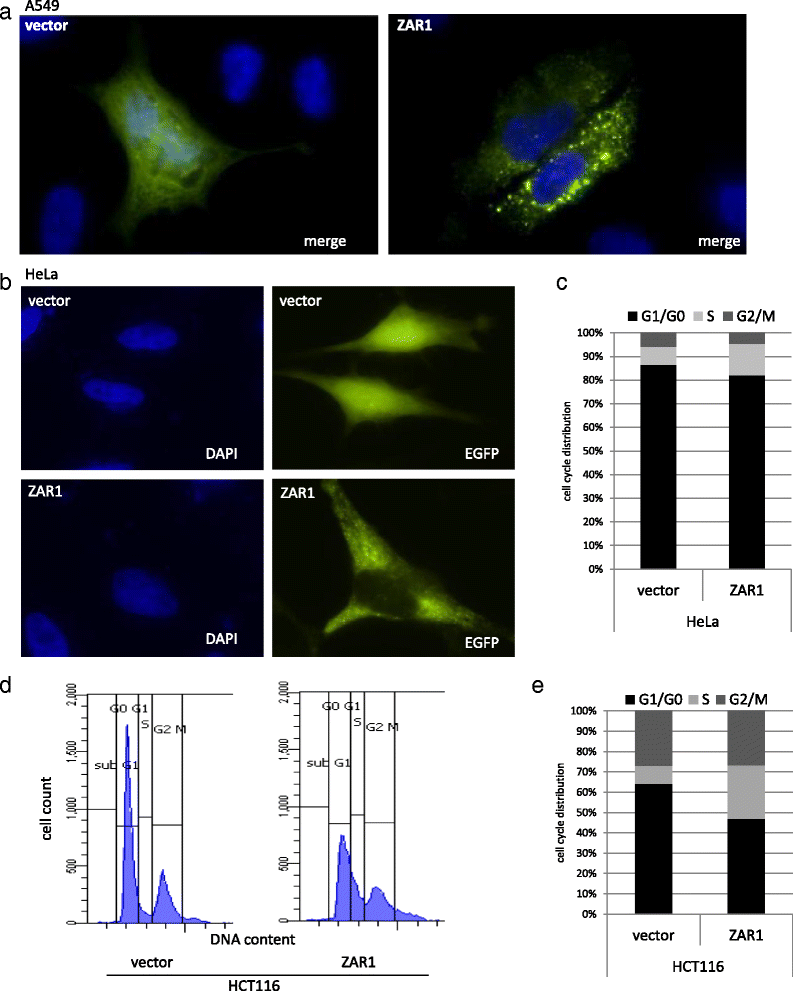
Results: In this study, we analysed ZAR1 (zygote arrest 1), which has been said to be a maternal-effect gene and its expression mostly limited to certain reproductive tissues. Our study shows that ZAR1 is expressed in normal lung but inactivated by promoter methylation in lung cancer. ZAR1 is hypermethylated in primary lung cancer samples (22% small cell lung carcinoma (SCLC) and 76% non-small cell lung carcinoma (NSCLC), p < 0.001) vs. normal control lung tissue (11%). In lung cancer cell lines, ZAR1 was significantly methylated in 75% of SCLC and 83% of NSCLC vs. normal tissue (p < 0.005/0.05). In matching tumours and control tissues, we observed that NSCLC primary tumour samples exhibited a tumour-specific promoter methylation of ZAR1 in comparison to the normal control lung tissue. Demethylation treatment of various lung cancer cell lines reversed ZAR1 promoter hypermethylation and subsequently re-established ZAR1 expression. In addition, we could show the growth inhibitory potential of ZAR1 in lung cancer cell lines and cancer cell lines. Exogenous expression of ZAR1 not only inhibited colony formation but also blocked cell cycle progression of cancer cell lines.
Conclusions: Our study shows for the first time the lung tumour-specific epigenetic inactivation of ZAR1 due to DNA methylation of its CpG island promoter. Furthermore, ZAR1 was characterised by the ability to block tumour growth through the inhibition of cell cycle progression in cancer cell lines. We propose that ZAR1 could serve as an epigenetically inactivated biomarker in lung cancer.
Keywords: DNA methylation; Epigenetics; Lung cancer; Tumour suppressor; ZAR1 (zygote arrest 1).
Figures


Similar articles
-
Epigenetic therapy of novel tumour suppressor ZAR1 and its cancer biomarker function.Clin Epigenetics. 2019 Dec 4;11(1):182. doi: 10.1186/s13148-019-0774-2. Clin Epigenetics. 2019. PMID: 31801617 Free PMC article.
-
The ZAR1 protein in cancer; from epigenetic silencing to functional characterisation and epigenetic therapy of tumour suppressors.Biochim Biophys Acta Rev Cancer. 2020 Dec;1874(2):188417. doi: 10.1016/j.bbcan.2020.188417. Epub 2020 Aug 21. Biochim Biophys Acta Rev Cancer. 2020. PMID: 32828887 Review.
-
Paired box 5 is a frequently methylated lung cancer tumour suppressor gene interfering β-catenin signalling and GADD45G expression.J Cell Mol Med. 2016 May;20(5):842-54. doi: 10.1111/jcmm.12768. Epub 2016 Feb 4. J Cell Mol Med. 2016. PMID: 26843424 Free PMC article.
-
Aberrant Promoter Methylation of the Tumour Suppressor RASSF10 and Its Growth Inhibitory Function in Breast Cancer.Cancers (Basel). 2016 Feb 25;8(3):26. doi: 10.3390/cancers8030026. Cancers (Basel). 2016. PMID: 26927176 Free PMC article.
-
Non-promoter hypermethylation of zygote arrest 1 (ZAR1) in human brain tumors.Brain Tumor Pathol. 2011 Jul;28(3):199-202. doi: 10.1007/s10014-011-0019-3. Epub 2011 Feb 18. Brain Tumor Pathol. 2011. PMID: 21331615 Review.
Cited by
-
Sex-Specific Transcriptome Differences in Human Adipose Mesenchymal Stem Cells.Genes (Basel). 2020 Aug 8;11(8):909. doi: 10.3390/genes11080909. Genes (Basel). 2020. PMID: 32784482 Free PMC article.
-
The Relevance of Gender in Tumor-Influencing Epigenetic Traits.Epigenomes. 2019 Jan 28;3(1):6. doi: 10.3390/epigenomes3010006. Epigenomes. 2019. PMID: 34991275 Free PMC article. Review.
-
Long Noncoding RNA Urothelial Carcinoma-Associated 1 Promotes the Proliferation and Metastasis of Human Lung Tumor Cells by Regulating MicroRNA-144.Oncol Res. 2018 May 7;26(4):537-546. doi: 10.3727/096504017X15009792179602. Epub 2017 Aug 1. Oncol Res. 2018. Retraction in: Oncol Res. 2024 Oct 16;32(11):1825. doi: 10.32604/or.2024.056897. PMID: 28762326 Free PMC article. Retracted.
-
ZAR1/2-Regulated Epigenetic Modifications are Essential for Age-Associated Oocyte Quality Maintenance and Zygotic Activation.Adv Sci (Weinh). 2025 Feb;12(8):e2410305. doi: 10.1002/advs.202410305. Epub 2025 Jan 4. Adv Sci (Weinh). 2025. PMID: 39755931 Free PMC article.
-
Hepatitis B virus impacts embryonic development and methylation of maternal genes in assisted reproductive technology patients.J Assist Reprod Genet. 2025 Mar;42(3):809-815. doi: 10.1007/s10815-024-03359-4. Epub 2024 Dec 27. J Assist Reprod Genet. 2025. PMID: 39730946 Free PMC article.
References
-
- Shi L, Zheng M, Hou J, Zhu B, Wang X. Regulatory roles of epigenetic modulators, modifiers and mediators in lung cancer. Semin Cancer Biol. 2016. Epub 2016/11/15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

