Going beyond personal protection against mosquito bites to eliminate malaria transmission: population suppression of malaria vectors that exploit both human and animal blood
- PMID: 28589015
- PMCID: PMC5444054
- DOI: 10.1136/bmjgh-2016-000198
Going beyond personal protection against mosquito bites to eliminate malaria transmission: population suppression of malaria vectors that exploit both human and animal blood
Abstract
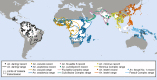
Protecting individuals and households against mosquito bites with long-lasting insecticidal nets (LLINs) or indoor residual spraying (IRS) can suppress entire populations of unusually efficient malaria vector species that predominantly feed indoors on humans. Mosquitoes which usually feed on animals are less reliant on human blood, so they are far less vulnerable to population suppression effects of such human-targeted insecticidal measures. Fortunately, the dozens of mosquito species which primarily feed on animals are also relatively inefficient vectors of malaria, so personal protection against mosquito bites may be sufficient to eliminate transmission. However, a handful of mosquito species are particularly problematic vectors of residual malaria transmission, because they feed readily on both humans and animals. These unusual vectors feed often enough on humans to be potent malaria vectors, but also often enough on animals to evade population control with LLINs, IRS or any other insecticidal personal protection measure targeted only to humans. Anopheles arabiensis and A. coluzzii in Africa, A. darlingi in South America and A. farauti in Oceania, as well as A. culicifacies species E, A. fluviatilis species S, A. lesteri and A. minimus in Asia, all feed readily on either humans or animals and collectively mediate residual malaria transmission across most of the tropics. Eliminating malaria transmission by vectors exhibiting such dual host preferences will require aggressive mosquito population abatement, rather than just personal protection of humans. Population suppression of even these particularly troublesome vectors is achievable with a variety of existing vector control technologies that remain underdeveloped or underexploited.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Kiswewski A, Mellinger A, Spielman A, et al. . A global index representing the stability of malaria transmission. Am J Trop Med Hyg 2004;70:486–98. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
