Radiation-Induced Oral Mucositis
- PMID: 28589080
- PMCID: PMC5439125
- DOI: 10.3389/fonc.2017.00089
Radiation-Induced Oral Mucositis
Abstract
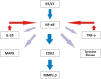
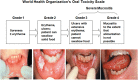
Radiation-induced oral mucositis (RIOM) is a major dose-limiting toxicity in head and neck cancer patients. It is a normal tissue injury caused by radiation/radiotherapy (RT), which has marked adverse effects on patient quality of life and cancer therapy continuity. It is a challenge for radiation oncologists since it leads to cancer therapy interruption, poor local tumor control, and changes in dose fractionation. RIOM occurs in 100% of altered fractionation radiotherapy head and neck cancer patients. In the United Sates, its economic cost was estimated to reach 17,000.00 USD per patient with head and neck cancers. This review will discuss RIOM definition, epidemiology, impact and side effects, pathogenesis, scoring scales, diagnosis, differential diagnosis, prevention, and treatment.
Keywords: chemotherapy; mesenchymal stromal/stem cells; normal tissue injury; oral mucositis; pathobiology; radiation; radiotherapy.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

