Immune System, Friend or Foe of Oncolytic Virotherapy?
- PMID: 28589085
- PMCID: PMC5440545
- DOI: 10.3389/fonc.2017.00106
Immune System, Friend or Foe of Oncolytic Virotherapy?
Abstract
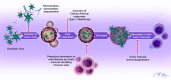
Oncolytic viruses (OVs) are an emerging class of targeted anticancer therapies designed to selectively infect, replicate in, and lyse malignant cells without causing harm to normal, healthy tissues. In addition to direct oncolytic activity, OVs have shown dual promise as immunotherapeutic agents. The presence of viral infection and subsequently generated immunogenic tumor cell death trigger innate and adaptive immune responses that mediate further tumor destruction. However, antiviral immune responses can intrinsically limit OV infection, spread, and overall therapeutic efficacy. Host immune system can act both as a barrier as well as a facilitator and sometimes both at the same time based on the phase of viral infection. Thus, manipulating the host immune system to minimize antiviral responses and viral clearance while still promoting immune-mediated tumor destruction remains a key challenge facing oncolytic virotherapy. Recent clinical trials have established the safety, tolerability, and efficacy of virotherapies in the treatment of a variety of malignancies. Most notably, talimogene laherparepvec (T-VEC), a genetically engineered oncolytic herpesvirus-expressing granulocyte macrophage colony stimulating factor, was recently approved for the treatment of melanoma, representing the first OV to be approved by the FDA as an anticancer therapy in the US. This review discusses OVs and their antitumor properties, their complex interactions with the immune system, synergy between virotherapy and existing cancer treatments, and emerging strategies to augment the efficacy of OVs as anticancer therapies.
Keywords: adaptive immunity; cancer; immunotherapy; innate immunity; oncolytic virus.
Figures

References
-
- Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med (2000) 6(8):879–85.10.1038/78638 - DOI - PubMed
-
- Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res (2003) 9:555–61. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

