Manipulation of Neutrophils by Porphyromonas gingivalis in the Development of Periodontitis
- PMID: 28589098
- PMCID: PMC5440471
- DOI: 10.3389/fcimb.2017.00197
Manipulation of Neutrophils by Porphyromonas gingivalis in the Development of Periodontitis
Abstract
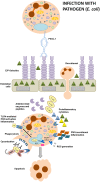
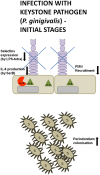
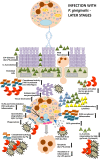
The pathogenesis of the chronic periodontal disease is associated with a skewed host inflammatory response to periodontal pathogens, such as Porphyromonas gingivalis, that accounts for the majority of periodontal tissue damage. Neutrophils are the most abundant leukocytes in periodontal pockets and depending on the stage of the disease, also plentiful PMNs are present in the inflamed gingival tissue and the gingival crevice. They are the most efficient phagocytes and eliminate pathogens by a variety of means, which are either oxygen-dependent or -independent. However, these secretory lethal weapons do not strictly discriminate between pathogens and host tissue. Current studies describe conflicting findings about neutrophil involvement in periodontal disease. On one hand literature indicate that hyper-reactive neutrophils are the main immune cell type responsible for this observed tissue damage and disease progression. Deregulation of neutrophil survival and functions, such as chemotaxis, migration, secretion of antimicrobial peptides or enzymes, and production of reactive oxygen species, contribute to observed tissue injury and the clinical signs of periodontal disease. On the other hand neutrophils deficiencies in patients and mice also result in periodontal phenotype. Therefore, P. gingivalis represents a periodontal pathogen that manipulates the immune responses of PMNs, employing several virulence factors, such as gingipains, serine proteases, lipid phosphatases, or fimbriae. This review will sum up studies devoted to understanding different strategies utilized by P. gingivalis to manipulate PMNs survival and functions in order to inhibit killing by a granular content, prolong inflammation, and gain access to nutrient resources.
Keywords: Porphyromonas gingivalis; inflammation; neutrophils; periodontitis; virulence factors.
Figures
Similar articles
-
The role of phagocytosis, oxidative burst and neutrophil extracellular traps in the interaction between neutrophils and the periodontal pathogen Porphyromonas gingivalis.Mol Oral Microbiol. 2015 Oct;30(5):361-75. doi: 10.1111/omi.12099. Epub 2015 May 4. Mol Oral Microbiol. 2015. PMID: 25869817
-
Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen.FEMS Microbiol Lett. 2012 Aug;333(1):1-9. doi: 10.1111/j.1574-6968.2012.02579.x. Epub 2012 May 28. FEMS Microbiol Lett. 2012. PMID: 22530835 Review.
-
Neutrophil-mediated host response to Porphyromonas gingivalis.J Int Acad Periodontol. 2002 Oct;4(4):119-25. J Int Acad Periodontol. 2002. PMID: 12670091 Review.
-
Inactive Gingipains from P. gingivalis Selectively Skews T Cells toward a Th17 Phenotype in an IL-6 Dependent Manner.Front Cell Infect Microbiol. 2017 Apr 27;7:140. doi: 10.3389/fcimb.2017.00140. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28497028 Free PMC article.
-
Porphyromonas gingivalis gingipains cause defective macrophage migration towards apoptotic cells and inhibit phagocytosis of primary apoptotic neutrophils.Cell Death Dis. 2017 Mar 2;8(3):e2644. doi: 10.1038/cddis.2016.481. Cell Death Dis. 2017. PMID: 28252646 Free PMC article.
Cited by
-
Genes Contributing to Porphyromonas gingivalis Fitness in Abscess and Epithelial Cell Colonization Environments.Front Cell Infect Microbiol. 2017 Aug 28;7:378. doi: 10.3389/fcimb.2017.00378. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28900609 Free PMC article.
-
Porphyromonas gingivalis induces penetration of lipopolysaccharide and peptidoglycan through the gingival epithelium via degradation of junctional adhesion molecule 1.PLoS Pathog. 2019 Nov 7;15(11):e1008124. doi: 10.1371/journal.ppat.1008124. eCollection 2019 Nov. PLoS Pathog. 2019. PMID: 31697789 Free PMC article.
-
Periodontitis pathogen Porphyromonas gingivalis promotes pancreatic tumorigenesis via neutrophil elastase from tumor-associated neutrophils.Gut Microbes. 2022 Jan-Dec;14(1):2073785. doi: 10.1080/19490976.2022.2073785. Gut Microbes. 2022. PMID: 35549648 Free PMC article.
-
Intratumour microbiome of pancreatic cancer.World J Gastrointest Oncol. 2023 May 15;15(5):713-730. doi: 10.4251/wjgo.v15.i5.713. World J Gastrointest Oncol. 2023. PMID: 37275446 Free PMC article. Review.
-
Neutrophils exhibit an individual response to different oral bacterial biofilms.J Oral Microbiol. 2020 Dec 9;13(1):1856565. doi: 10.1080/20002297.2020.1856565. J Oral Microbiol. 2020. PMID: 33391628 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

