Serine Protease Inhibitors in Ticks: An Overview of Their Role in Tick Biology and Tick-Borne Pathogen Transmission
- PMID: 28589099
- PMCID: PMC5438962
- DOI: 10.3389/fcimb.2017.00199
Serine Protease Inhibitors in Ticks: An Overview of Their Role in Tick Biology and Tick-Borne Pathogen Transmission
Abstract
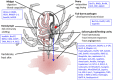
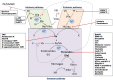
New tick and tick-borne pathogen control approaches that are both environmentally sustainable and which provide broad protection are urgently needed. Their development, however, will rely on a greater understanding of tick biology, tick-pathogen, and tick-host interactions. The recent advances in new generation technologies to study genomes, transcriptomes, and proteomes has resulted in a plethora of tick biomacromolecular studies. Among these, many enzyme inhibitors have been described, notably serine protease inhibitors (SPIs), whose importance in various tick biological processes is only just beginning to be fully appreciated. Among the multiple active substances secreted during tick feeding, SPIs have been shown to be directly involved in regulation of inflammation, blood clotting, wound healing, vasoconstriction and the modulation of host defense mechanisms. In light of these activities, several SPIs were examined and were experimentally confirmed to facilitate tick pathogen transmission. In addition, to prevent coagulation of the ingested blood meal within the tick alimentary canal, SPIs are also involved in blood digestion and nutrient extraction from the meal. The presence of SPIs in tick hemocytes and their involvement in tick innate immune defenses have also been demonstrated, as well as their implication in hemolymph coagulation and egg development. Considering the involvement of SPIs in multiple crucial aspects of tick-host-pathogen interactions, as well as in various aspects of the tick parasitic lifestyle, these molecules represent highly suitable and attractive targets for the development of effective tick control strategies. Here we review the current knowledge regarding this class of inhibitors in tick biology and tick-borne pathogen transmission, and their potential as targets for future tick control trials.
Keywords: immune responses; tick serine protease inhibitors; tick-borne pathogens; ticks; tick–host interactions.
Figures

Similar articles
-
Induced Transient Immune Tolerance in Ticks and Vertebrate Host: A Keystone of Tick-Borne Diseases?Front Immunol. 2021 Feb 12;12:625993. doi: 10.3389/fimmu.2021.625993. eCollection 2021. Front Immunol. 2021. PMID: 33643313 Free PMC article. Review.
-
Editorial: Tick-Host-Pathogen Interactions.Front Cell Infect Microbiol. 2018 Jun 15;8:194. doi: 10.3389/fcimb.2018.00194. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29963500 Free PMC article. No abstract available.
-
Protease Inhibitors in Tick Saliva: The Role of Serpins and Cystatins in Tick-host-Pathogen Interaction.Front Cell Infect Microbiol. 2017 May 29;7:216. doi: 10.3389/fcimb.2017.00216. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28611951 Free PMC article. Review.
-
Tick innate immunity.Adv Exp Med Biol. 2010;708:137-62. Adv Exp Med Biol. 2010. PMID: 21528697 Review.
-
Tick immunobiology.Parasitology. 2004;129 Suppl:S161-76. doi: 10.1017/s0031182004004834. Parasitology. 2004. PMID: 15940820 Review.
Cited by
-
Challenges for ticks and tick-borne diseases research in Southeast Asia: Insight from the first international symposium in Cambodia.PLoS Negl Trop Dis. 2024 Jul 10;18(7):e0012269. doi: 10.1371/journal.pntd.0012269. eCollection 2024 Jul. PLoS Negl Trop Dis. 2024. PMID: 38985826 Free PMC article. Review.
-
The immunosuppressive functions of two novel tick serpins, HlSerpin-a and HlSerpin-b, from Haemaphysalis longicornis.Immunology. 2020 Jan;159(1):109-120. doi: 10.1111/imm.13130. Epub 2019 Nov 10. Immunology. 2020. PMID: 31606893 Free PMC article.
-
Immunomodulatory effects of Rhipicephalus haemaphysaloides serpin RHS2 on host immune responses.Parasit Vectors. 2019 Jul 11;12(1):341. doi: 10.1186/s13071-019-3607-4. Parasit Vectors. 2019. PMID: 31296257 Free PMC article.
-
Immunomodulatory Proteins in Tick Saliva From a Structural Perspective.Front Cell Infect Microbiol. 2021 Oct 13;11:769574. doi: 10.3389/fcimb.2021.769574. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34722347 Free PMC article. Review.
-
N-glycoproteomic profiling reveals structural and functional alterations in yellow primary preserved egg white under saline-alkali treatment.Food Chem X. 2024 Feb 19;21:101244. doi: 10.1016/j.fochx.2024.101244. eCollection 2024 Mar 30. Food Chem X. 2024. PMID: 38420501 Free PMC article.
References
-
- Alim M. A., Islam M. K., Anisuzzaman, Miyoshi T., Hatta T., Yamaji K., et al. . (2012). A hemocyte-derived Kunitz–BPTI-type chymotrypsin inhibitor, HlChI, from the Ixodid tick Haemaphysalis longicornis, plays regulatory functions in tick blood-feeding processes. Insect Biochem. Mol. Biol. 42, 925–934. 10.1016/j.ibmb.2012.09.005 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

