Immune and myodegenerative pathomechanisms in inclusion body myositis
- PMID: 28589170
- PMCID: PMC5454400
- DOI: 10.1002/acn3.419
Immune and myodegenerative pathomechanisms in inclusion body myositis
Abstract
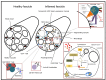
Inclusion Body Myositis (IBM) is a relatively common acquired inflammatory myopathy in patients above 50 years of age. Pathological hallmarks of IBM are intramyofiber protein inclusions and endomysial inflammation, indicating that both myodegenerative and inflammatory mechanisms contribute to its pathogenesis. Impaired protein degradation by the autophagic machinery, which regulates innate and adaptive immune responses, in skeletal muscle fibers has recently been identified as a potential key pathomechanism in IBM. Immunotherapies, which are successfully used for treating other inflammatory myopathies lack efficacy in IBM and so far no effective treatment is available. Thus, a better understanding of the mechanistic pathways underlying progressive muscle weakness and atrophy in IBM is crucial in identifying novel promising targets for therapeutic intervention. Here, we discuss recent insights into the pathomechanistic network of mutually dependent inflammatory and degenerative events during IBM.
Figures

References
-
- Dalakas MC. Polymyositis, dermatomyositis and inclusion‐body myositis. N Engl J Med 1991;325:1487–1498. - PubMed
-
- Dalakas MC. Inflammatory muscle diseases. N Engl J Med 2015;372:1734–1747. - PubMed
-
- Yunis EJ, Samaha FJ. Inclusion body myositis. Lab Invest 1971;25:240–248. - PubMed
-
- Schmidt J, Dalakas MC. Inclusion‐body myositis in the elderly: an update. Aging Health 2010;6:687–694. https://doi.org/10.2217/ahe.10.64;6(6):687-694. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

