Characterization of functional μ opioid receptor turnover in rat locus coeruleus: an electrophysiological and immunocytochemical study
- PMID: 28589556
- PMCID: PMC5522999
- DOI: 10.1111/bph.13901
Characterization of functional μ opioid receptor turnover in rat locus coeruleus: an electrophysiological and immunocytochemical study
Abstract
Background and purpose: Regulation of μ receptor dynamics such as its trafficking is a possible mechanism underlying opioid tolerance that contributes to inefficient recycling of opioid responses. We aimed to characterize the functional turnover of μ receptors in the noradrenergic nucleus locus coeruleus (LC).
Experimental approach: We measured opioid effect by single-unit extracellular recordings of LC neurons from rat brain slices. Immunocytochemical techniques were used to evaluate μ receptor trafficking.
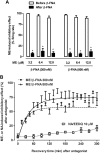
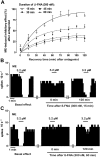
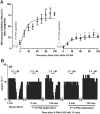
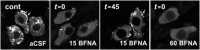
Key results: After near-complete, irreversible μ receptor inactivation with β-funaltrexamine (β-FNA), opioid effect spontaneously recovered in a rapid and efficacious manner. In contrast, α2 -adrenoceptor-mediated effect hardly recovered after receptor inactivation with the irreversible antagonist EEDQ. When the recovery of opioid effect was tested after various inactivating time schedules, we found that the longer the β-FNA pre-exposure, the less efficient and slower the functional μ receptor turnover became. Interestingly, μ receptor turnover was slower when β-FNA challenge was repeated in the same cell, indicating constitutive μ receptor recycling by trafficking from a depletable pool. Double immunocytochemistry confirmed the constitutive nature of μ receptor trafficking from a cytoplasmic compartment. The μ receptor turnover was slowed down when LC neuron calcium- or firing-dependent processes were prevented or vesicular protein trafficking was blocked by a low temperature or transport inhibitor.
Conclusions and implications: Constitutive trafficking of μ receptors from a depletable intracellular pool (endosome) may account for its rapid and efficient functional turnover in the LC. A finely-tuned regulation of μ receptor trafficking and endosomes could explain neuroadaptive plasticity to opioids in the LC.
© 2017 The British Pharmacological Society.
Figures


References
-
- Adler CH, Meller E, Goldstein M (1985). Recovery of alpha 2‐adrenoceptor binding and function after irreversible inactivation by N‐ethoxycarbonyl‐2‐ethoxy‐1, 2‐dihydroquinoline (EEDQ). Eur J Pharmacol 116: 175–178. - PubMed
-
- Aghajanian GK, Wang YY (1987). Common alpha 2‐ and opiate effector mechanisms in the locus coeruleus: intracellular studies in brain slices. Neuropharmacology 26: 793–799. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

