Timing and Characteristics of Cumulative Evidence Available on Novel Therapeutic Agents Receiving Food and Drug Administration Accelerated Approval
- PMID: 28589600
- PMCID: PMC5461381
- DOI: 10.1111/1468-0009.12261
Timing and Characteristics of Cumulative Evidence Available on Novel Therapeutic Agents Receiving Food and Drug Administration Accelerated Approval
Abstract
Policy Points: Randomized trials-the gold standard of evaluating effectiveness-constitute a small minority of existing evidence on agents given accelerated approval. One-third of randomized trials are in therapeutic areas outside of FDA approval and less than half evaluate the therapeutic benefits of these agents but use them instead as common backbone treatments. Agents receiving accelerated approval are often tested concurrently in several therapeutic areas. For most agents, no substantial time lag is apparent between the average start dates of randomized trials evaluating their effectiveness and those using them as part of background therapies. There appears to be a tendency for therapeutic agents receiving accelerated approval to quickly become an integral component of standard treatment, despite potential shortcomings in their evidence base.
Context: Therapeutic agents treating serious conditions are eligible for Food and Drug Administration (FDA) accelerated approval. The clinical evidence accrued on agents receiving accelerated approval has not been systematically evaluated. Our objective was to assess the timing and characteristics of available studies.
Methods: We first identified clinical studies of novel therapeutic agents receiving accelerated approval. We then (1) categorized those studies as randomized or nonrandomized, (2) explored whether they evaluated the FDA-approved indications, and (3) documented the available treatment comparisons. We also meta-analyzed the difference in start times between randomized studies that (1) did or did not evaluate approved indications and (2) were or were not designed to evaluate the agent's effectiveness.
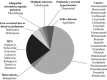
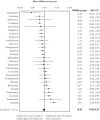
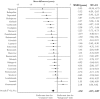
Findings: In total, 37 novel therapeutic agents received accelerated approval between 2000 and 2013. Our search of ClinicalTrials.gov identified 7,757 studies, which included 1,258,315 participants. Only one-third of identified studies were randomized controlled trials. Of 1,631 randomized trials with advanced recruitment status, 906 were conducted in therapeutic areas for which agents received initial accelerated approval, 202 were in supplemental indications, and 523 were outside approved indications. Only 411 out of 906 (45.4%) trials were designed to test the effectiveness of agents that received accelerated approval ("evaluation" trials); others used these agents as common background treatment in both arms ("background" trials). There was no detectable lag between average start times of trials conducted within and outside initially approved indications. Evaluation trials started on average 1.52 years (95% CI: 0.87 to 2.17) earlier than background trials.
Conclusions: Cumulative evidence on agents with accelerated approvals has major limitations. Most clinical studies including these agents are small and nonrandomized, and about a third are conducted in unapproved areas, typically concurrently with those conducted in approved areas. Most randomized trials including these therapeutic agents are not designed to directly evaluate their clinical benefits but to incorporate them as standard treatment.
Keywords: Food and Drug Administration; accelerated approval; market authorization; pharmaceutical policy.
© 2017 Milbank Memorial Fund.
Figures


Similar articles
-
The US Food and Drug Administration's expedited approval programs: Evidentiary standards, regulatory trade-offs, and potential improvements.Clin Trials. 2018 Jun;15(3):219-229. doi: 10.1177/1740774518770648. Clin Trials. 2018. PMID: 29871509
-
Clinical Trial Evidence Supporting US Food and Drug Administration Approval of Novel Cancer Therapies Between 2000 and 2016.JAMA Netw Open. 2020 Nov 2;3(11):e2024406. doi: 10.1001/jamanetworkopen.2020.24406. JAMA Netw Open. 2020. PMID: 33170262 Free PMC article.
-
Approval of Cancer Drugs With Uncertain Therapeutic Value: A Comparison of Regulatory Decisions in Europe and the United States.Milbank Q. 2020 Dec;98(4):1219-1256. doi: 10.1111/1468-0009.12476. Epub 2020 Oct 6. Milbank Q. 2020. PMID: 33021339 Free PMC article.
-
Accelerated approval of oncology products: the food and drug administration experience.J Natl Cancer Inst. 2011 Apr 20;103(8):636-44. doi: 10.1093/jnci/djr062. Epub 2011 Mar 21. J Natl Cancer Inst. 2011. PMID: 21422403 Review.
-
Accelerated approval of oncology products: a decade of experience.J Natl Cancer Inst. 2004 Oct 20;96(20):1500-9. doi: 10.1093/jnci/djh279. J Natl Cancer Inst. 2004. PMID: 15494600 Review.
Cited by
-
HTA Barriers for Conditional Approval Drugs.Pharmacoeconomics. 2023 May;41(5):529-545. doi: 10.1007/s40273-023-01248-9. Epub 2023 Feb 23. Pharmacoeconomics. 2023. PMID: 36821044
-
Analysis of Postapproval Clinical Trials of Therapeutics Approved by the US Food and Drug Administration Without Clinical Postmarketing Requirements or Commitments.JAMA Netw Open. 2019 May 3;2(5):e193410. doi: 10.1001/jamanetworkopen.2019.3410. JAMA Netw Open. 2019. PMID: 31074812 Free PMC article.
-
Characteristics of Preapproval and Postapproval Studies for Drugs Granted Accelerated Approval by the US Food and Drug Administration.JAMA. 2017 Aug 15;318(7):626-636. doi: 10.1001/jama.2017.9415. JAMA. 2017. PMID: 28810023 Free PMC article.
-
Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13.BMJ. 2017 Oct 4;359:j4530. doi: 10.1136/bmj.j4530. BMJ. 2017. PMID: 28978555 Free PMC article.
-
US Food and Drug Administration Accelerated Approval Program for Nononcology Drug Indications Between 1992 and 2018.JAMA Netw Open. 2022 Sep 1;5(9):e2230973. doi: 10.1001/jamanetworkopen.2022.30973. JAMA Netw Open. 2022. PMID: 36083581 Free PMC article.
References
-
- Carpenter D. Reputation and Power: Organizational Image and Pharmaceutical Regulation at the FDA. Princeton, NJ: Princeton University Press; 2014.
-
- US Food and Drug Administration . Guidance for Industry: Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products. Washington, DC: US Food and Drug Administration; 1998. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatory Information/Gui.... Accessed March 21, 2017.
-
- Kesselheim AS, Darrow JJ. FDA designations for therapeutics and their impact on drug development and regulatory review outcomes. Clin Pharmacol Ther. 2015;97(1):29‐36. - PubMed
-
- US Food and Drug Administration . Guidance for Industry: Expedited Programs for Serious Conditions—Drugs and Biologics. US Food and Drug Administration: Washington, DC; 2014. www.fda.gov/downloads/drugs/guidancecomplianceregulatory information/gui.... Accessed March 21, 2017.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

