Dendro-dendritic cholinergic excitation controls dendritic spike initiation in retinal ganglion cells
- PMID: 28589928
- PMCID: PMC5477517
- DOI: 10.1038/ncomms15683
Dendro-dendritic cholinergic excitation controls dendritic spike initiation in retinal ganglion cells
Abstract
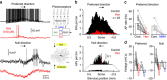
The retina processes visual images to compute features such as the direction of image motion. Starburst amacrine cells (SACs), axonless feed-forward interneurons, are essential components of the retinal direction-selective circuitry. Recent work has highlighted that SAC-mediated dendro-dendritic inhibition controls the action potential output of direction-selective ganglion cells (DSGCs) by vetoing dendritic spike initiation. However, SACs co-release GABA and the excitatory neurotransmitter acetylcholine at dendritic sites. Here we use direct dendritic recordings to show that preferred direction light stimuli evoke SAC-mediated acetylcholine release, which powerfully controls the stimulus sensitivity, receptive field size and action potential output of ON-DSGCs by acting as an excitatory drive for the initiation of dendritic spikes. Consistent with this, paired recordings reveal that the activation of single ON-SACs drove dendritic spike generation, because of predominate cholinergic excitation received on the preferred side of ON-DSGCs. Thus, dendro-dendritic release of neurotransmitters from SACs bi-directionally gate dendritic spike initiation to control the directionally selective action potential output of retinal ganglion cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures









References
-
- Denk W., Briggman K. L. & Helmstaedter M. Structural neurobiology: missing link to a mechanistic understanding of neural computation. Nat. Rev. Neurosci. 13, 351–358 (2012). - PubMed
-
- Oyster C. W. & Barlow H. B. Direction-selective units in rabbit retina: distribution of preferred directions. Science 155, 841–842 (1967). - PubMed
-
- Borst A. & Euler T. Seeing things in motion: models, circuits, and mechanisms. Neuron 71, 974–994 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

