Dynamic alterations in decoy VEGF receptor-1 stability regulate angiogenesis
- PMID: 28589930
- PMCID: PMC5467243
- DOI: 10.1038/ncomms15699
Dynamic alterations in decoy VEGF receptor-1 stability regulate angiogenesis
Abstract
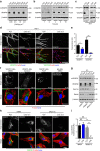
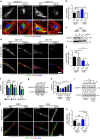
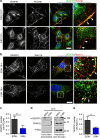
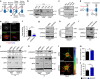
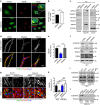
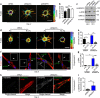
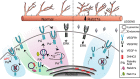
Blood vessel expansion is driven by sprouting angiogenesis of endothelial cells, and is essential for development, wound healing and disease. Membrane-localized vascular endothelial growth factor receptor-1 (mVEGFR1) is an endothelial cell-intrinsic decoy receptor that negatively modulates blood vessel morphogenesis. Here we show that dynamic regulation of mVEGFR1 stability and turnover in blood vessels impacts angiogenesis. mVEGFR1 is highly stable and constitutively internalizes from the plasma membrane. Post-translational palmitoylation of mVEGFR1 is a binary stabilization switch, and ligand engagement leads to depalmitoylation and lysosomal degradation. Trafficking of palmitoylation enzymes via Rab27a regulates mVEGFR1 stability, as reduced levels of Rab27a impaired palmitoylation of mVEGFR1, decreased its stability, and elevated blood vessel sprouting and in vivo angiogenesis. These findings identify a regulatory axis affecting blood vessel morphogenesis that highlights exquisite post-translational regulation of mVEGFR1 in its role as a molecular rheostat.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Adams R. H. & Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. rev. Mol. Cell Biol. 8, 464–478 (2007). - PubMed
-
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature 438, 932–936 (2005). - PubMed
-
- Potente M., Gerhardt H. & Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011). - PubMed
-
- Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. Exs 94, 209–231 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

