Elevated prostaglandin E2 post-bone marrow transplant mediates interleukin-1β-related lung injury
- PMID: 28589946
- PMCID: PMC5720939
- DOI: 10.1038/mi.2017.51
Elevated prostaglandin E2 post-bone marrow transplant mediates interleukin-1β-related lung injury
Abstract
Hematopoietic stem cell transplant (HSCT) treats or cures a variety of hematological and inherited disorders. Unfortunately, patients who undergo HSCT are susceptible to infections by a wide array of opportunistic pathogens. Pseudomonas aeruginosa bacteria can have life-threatening effects in HSCT patients by causing lung pathology that has been linked to high levels of the potent pro-inflammatory cytokine, interleukin-1β (IL-1β). Using a murine bone marrow transplant (BMT) model, we show that overexpression of prostaglandin E2 (PGE2) post-BMT signals via EP2 or EP4 to induce cyclic adenosine monophosphate (cAMP), which activates protein kinase A or the exchange protein activated by cAMP (Epac) to induce cAMP response element binding-dependent transcription of IL-1β leading to exacerbated lung injury in BMT mice. Induction of IL-1β by PGE2 is time and dose dependent. Interestingly, IL-1β processing post-P. aeruginosa infection occurs via the enzymatic activity of either caspase-1 or caspase-8. Furthermore, PGE2 can limit autophagy-mediated killing of P. aeruginosa in alveolar macrophages, yet autophagy does not have a role in PGE2-mediated upregulation of IL-1β. Reducing PGE2 levels with indomethacin improved bacterial clearance and reduced IL-1β-mediated acute lung injury in P. aeruginosa-infected BMT mice.
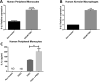
Figures
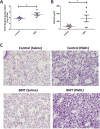

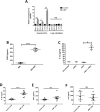

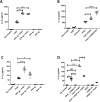


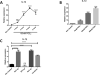

References
-
- Ullah K, Raza S, Ahmed P, Chaudhry QU, Satti TM, Ahmed S, et al. Post-transplant infections: single center experience from the developing world. Int J Infect Dis. 2008;12(2):203–214. - PubMed
-
- Wonnenberg B, Bischoff M, Beisswenger C, Dinh T, Bals R, Singh B, et al. The role of IL-1beta in Pseudomonas aeruginosa in lung infection. Cell Tissue Res. 2016;364(2):225–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

