Mycelium-mediated transfer of water and nutrients stimulates bacterial activity in dry and oligotrophic environments
- PMID: 28589950
- PMCID: PMC5467244
- DOI: 10.1038/ncomms15472
Mycelium-mediated transfer of water and nutrients stimulates bacterial activity in dry and oligotrophic environments
Abstract
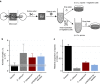
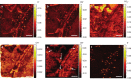
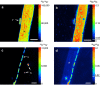
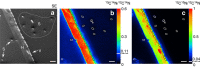
Fungal-bacterial interactions are highly diverse and contribute to many ecosystem processes. Their emergence under common environmental stress scenarios however, remains elusive. Here we use a synthetic microbial ecosystem based on the germination of Bacillus subtilis spores to examine whether fungal and fungal-like (oomycete) mycelia reduce bacterial water and nutrient stress in an otherwise dry and nutrient-poor microhabitat. We find that the presence of mycelia enables the germination and subsequent growth of bacterial spores near the hyphae. Using a combination of time of flight- and nanoscale secondary ion mass spectrometry (ToF- and nanoSIMS) coupled with stable isotope labelling, we link spore germination to hyphal transfer of water, carbon and nitrogen. Our study provides direct experimental evidence for the stimulation of bacterial activity by mycelial supply of scarce resources in dry and nutrient-free environments. We propose that mycelia may stimulate bacterial activity and thus contribute to sustaining ecosystem functioning in stressed habitats.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Wargo M. J. & Hogan D. A. Fungal—bacterial interactions: a mixed bag of mingling microbes. Curr. Opin. Microbiol. 9, 359–364 (2006). - PubMed
-
- Boer W. d., Folman L. B., Summerbell R. C. & Boddy L. Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 29, 795–811 (2005). - PubMed
-
- Pande S. et al.. Metabolic cross-feeding via intercellular nanotubes among bacteria. Nat. Commun. 6, 6238 (2015). - PubMed
-
- Lennon J. T. & Jones S. E. Microbial seed banks: the ecological and evolutionary implications of dormancy. Nat. Rev. Microbiol. 9, 119–130 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

