Monitoring focal adhesion kinase phosphorylation dynamics in live cells
- PMID: 28589989
- PMCID: PMC5531600
- DOI: 10.1039/c7an00471k
Monitoring focal adhesion kinase phosphorylation dynamics in live cells
Abstract
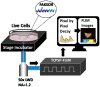
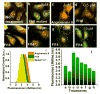
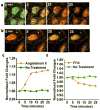
Focal adhesion kinase (FAK) is a cytoplasmic non-receptor tyrosine kinase essential for a diverse set of cellular functions. Current methods for monitoring FAK activity in response to an extracellular stimulus lack spatiotemporal resolution and/or the ability to perform multiplex detection. Here we report on a novel approach to monitor the real-time kinase phosphorylation activity of FAK in live single cells by fluorescence lifetime imaging.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Kritikou E. Nature reviews. Molecular cell biology. 2009;10:3–3.
-
- Farge E. Current topics in developmental biology. 2011;95:243–265. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

