Structure-function relationships at the human spinal disc-vertebra interface
- PMID: 28590060
- PMCID: PMC5720932
- DOI: 10.1002/jor.23627
Structure-function relationships at the human spinal disc-vertebra interface
Abstract
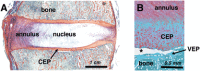
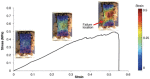
Damage at the intervertebral disc-vertebra interface associates with back pain and disc herniation. However, the structural and biomechanical properties of the disc-vertebra interface remain underexplored. We sought to measure mechanical properties and failure mechanisms, quantify architectural features, and assess structure-function relationships at this vulnerable location. Vertebra-disc-vertebra specimens from human cadaver thoracic spines were scanned with micro-computed tomography (μCT), surface speckle-coated, and loaded to failure in uniaxial tension. Digital image correlation (DIC) was used to calculate local surface strains. Failure surfaces were scanned using scanning electron microscopy (SEM), and adjacent sagittal slices were analyzed with histology and SEM. Seventy-one percent of specimens failed initially at the cartilage endplate-bone interface of the inner annulus region. Histology and SEM both indicated a lack of structural integration between the cartilage endplate (CEP) and bone. The interface failure strength was increased in samples with higher trabecular bone volume fraction in the vertebral endplates. Furthermore, failure strength decreased with degeneration, and in discs with thicker CEPs. Our findings indicate that poor structural connectivity between the CEP and vertebra may explain the structural weakness at this region, and provide insight into structural features that may contribute to risk for disc-vertebra interface injury. The disc-vertebra interface is the site of failure in the majority of herniation injuries. Here we show new structure-function relationships at this interface that may motivate the development of diagnostics, prevention strategies, and treatments to improve the prognosis for many low back pain patients with disc-vertebra interface injuries. © 2017 The Authors. Journal of Orthopaedic Research® Published by Wiley Periodicals, Inc. on behalf of Orthopaedic Research Society. J Orthop Res 36:192-201, 2018.
Keywords: avulsion; cartilage endplate junction; collagen; disc herniation; intervertebral disc.
© 2017 The Authors. Journal of Orthopaedic Research® Published by Wiley Periodicals, Inc. on behalf of Orthopaedic Research Society.
Figures









References
-
- Rajasekaran S, Bajaj N, Tubaki V, et al. 2013. ISSLS Prize winner: the anatomy of failure in lumbar disc herniation: an in vivo, multimodal, prospective study of 181 subjects. Spine (Phila Pa 1976) 38:1491–1500. - PubMed
-
- Lama P, Zehra U, Balkovec C, et al. 2014. Significance of cartilage endplate within herniated disc tissue. Eur Spine J 23:1869–1877. - PubMed
-
- Thompson KJ, Dagher AP, Eckel TS, et al. 2009. Modic changes on MR images as studied with provocative diskography: clinical relevance—a retrospective study of 2457 disks. Radiology 250:849–855. - PubMed
-
- Thomopoulos S, Williams GR, Gimbel J a, et al. 2003. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res 21:413–419. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

