Merotelic kinetochore attachment in oocyte meiosis II causes sister chromatids segregation errors in aged mice
- PMID: 28590163
- PMCID: PMC5553406
- DOI: 10.1080/15384101.2017.1327488
Merotelic kinetochore attachment in oocyte meiosis II causes sister chromatids segregation errors in aged mice
Abstract
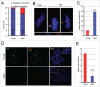
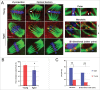
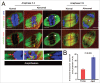
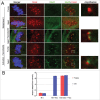
Mammalian oocyte chromosomes undergo 2 meiotic divisions to generate haploid gametes. The frequency of chromosome segregation errors during meiosis I increase with age. However, little attention has been paid to the question of how aging affects sister chromatid segregation during oocyte meiosis II. More importantly, how aneuploid metaphase II (MII) oocytes from aged mice evade the spindle assembly checkpoint (SAC) mechanism to complete later meiosis II to form aneuploid embryos remains unknown. Here, we report that MII oocytes from naturally aged mice exhibited substantial errors in chromosome arrangement and configuration compared with young MII oocytes. Interestingly, these errors in aged oocytes had no impact on anaphase II onset and completion as well as 2-cell formation after parthenogenetic activation. Further study found that merotelic kinetochore attachment occurred more frequently and could stabilize the kinetochore-microtubule interaction to ensure SAC inactivation and anaphase II onset in aged MII oocytes. This orientation could persist largely during anaphase II in aged oocytes, leading to severe chromosome lagging and trailing as well as delay of anaphase II completion. Therefore, merotelic kinetochore attachment in oocyte meiosis II exacerbates age-related genetic instability and is a key source of age-dependent embryo aneuploidy and dysplasia.
Keywords: aging; cohesion; kinetochore-microtubule attachment; meiosis II; oocytes; sister chromatids.
Figures
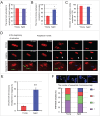
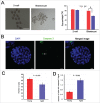

References
-
- Jones KT, Lane SI. Molecular causes of aneuploidy in mammalian eggs. Development 2013; 140:3719-30; PMID:23981655; https://doi.org/ 10.1242/dev.090589 - DOI - PubMed
-
- Chiang T, Schultz RM, Lampson MA. Meiotic origins of maternal age-related aneuploidy. Biol Reprod 2012; 86:1-7; PMID:21957193; https://doi.org/ 10.1095/biolreprod.111.094367 - DOI - PMC - PubMed
-
- Holt JE, Lane SI, Jones KT. The control of meiotic maturation in mammalian oocytes. Curr Top Dev Biol 2013; 102:207-26; PMID:23287034 - PubMed
-
- Yun Y, Lane SI, Jones KT. Premature dyad separation in meiosis II is the major segregation error with maternal age in mouse oocytes. Development 2014; 141:199-208; PMID:24346700; https://doi.org/ 10.1242/dev.100206 - DOI - PMC - PubMed
-
- Salmon ED, Cimini D, Cameron LA, Deluca JG. Merotelic kinetochores in mammalian tissue cells. Philos Trans R Soc Lond B Biol Sci 2005; 360:553-68; PMID:15897180; https://doi.org/ 10.1098/rstb.2004.1610 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
