A Subregion of the Parabrachial Nucleus Partially Mediates Respiratory Rate Depression from Intravenous Remifentanil in Young and Adult Rabbits
- PMID: 28590302
- PMCID: PMC5561451
- DOI: 10.1097/ALN.0000000000001719
A Subregion of the Parabrachial Nucleus Partially Mediates Respiratory Rate Depression from Intravenous Remifentanil in Young and Adult Rabbits
Abstract
Background: The efficacy of opioid administration to reduce postoperative pain is limited by respiratory depression. We investigated whether clinically relevant opioid concentrations altered the respiratory pattern in the parabrachial nucleus, a pontine region contributing to respiratory pattern generation, and compared these effects with a medullary respiratory site, the pre-Bötzinger complex.
Methods: Studies were performed in 40 young and 55 adult artificially ventilated, decerebrate rabbits. We identified an area in the parabrachial nucleus where α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid microinjections elicited tachypnea. Two protocols were performed in separate sets of animals. First, bilateral microinjections of the μ-opioid receptor agonist [D-Ala, N-MePhe, Gly-ol]-enkephalin (100 μM) into the "tachypneic area" determined the effect of maximal μ-opioid receptor activation. Second, respiratory rate was decreased with continuous IV infusions of remifentanil. The opioid antagonist naloxone (1 mM) was then microinjected bilaterally into the "tachypneic area" of the parabrachial nucleus to determine whether the respiratory rate depression could be locally reversed.
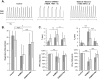
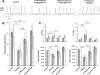
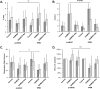
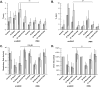
Results: Average respiratory rate was 27 ± 10 breaths/min. First, [D-Ala, N-MePhe, Gly-ol]-enkephalin injections decreased respiratory rate by 62 ± 20% in young and 45 ± 26% in adult rabbits (both P < 0.001). Second, during IV remifentanil infusion, bilateral naloxone injections into the "tachypneic area" of the parabrachial nucleus reversed respiratory rate depression from 55 ± 9% to 20 ± 14% in young and from 46 ± 20% to 18 ± 27% in adult rabbits (both P < 0.001). The effects of bilateral [D-Ala, N-MePhe, Gly-ol]-enkephalin injection and IV remifentanil on respiratory phase duration in the "tachypneic area" of the parabrachial nucleus was significantly different from the pre-Bötzinger complex.
Conclusions: The "tachypneic area" of the parabrachial nucleus is highly sensitive to μ-opioid receptor activation and mediates part of the respiratory rate depression by clinically relevant administration of opioids.
Conflict of interest statement
The authors state no conflicts of interest.
Figures
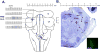
References
-
- Dahan A, Aarts L, Smith TW. Incidence, Reversal, and Prevention of Opioid-induced Respiratory Depression. Anesthesiology. 2010;112:226–38. - PubMed
-
- Hanna MH, Elliott KM, Fung M. Randomized, double-blind study of the analgesic efficacy of morphine-6-glucuronide versus morphine sulfate for postoperative pain in major surgery. Anesthesiology. 2005;102:815–21. - PubMed
-
- Lalley PM. Opiate slowing of feline respiratory rhythm and effects on putative medullary phase-regulating neurons. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1387–96. - PubMed
-
- Dahan A, Romberg R, Teppema L, Sarton E, Bijl H, Olofsen E. Simultaneous measurement and integrated analysis of analgesia and respiration after an intravenous morphine infusion. Anesthesiology. 2004;101:1201–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

