Comparison of the Effects of a Pharmaceutical Industry Decision Guide and Decision Aids on Patient Choice to Intensify Therapy in Rheumatoid Arthritis
- PMID: 28590834
- PMCID: PMC5466141
- DOI: 10.1177/0272989X17696995
Comparison of the Effects of a Pharmaceutical Industry Decision Guide and Decision Aids on Patient Choice to Intensify Therapy in Rheumatoid Arthritis
Abstract
Objective: To compare the effects a pharmaceutical industry decision guide and International Patient Decision Aids Standard (IPDAS) compliant patient decision aids (PtDA) on patient medication beliefs and choice to intensify therapy.
Methods: Rheumatoid arthritis (RA) patients, who had never taken etanercept (Enbrel), took part in a mail survey. They were presented with a hypothetical decision scenario where they were asked to consider adding etanercept to their current regimen. Each patient was randomized to review 1 of 3 forms of an etanercept-specific decision support: a long PtDA (LONG DA), a short PtDA (SHORT DA), or the manufacturer's Enbrel decision guide (Pharm Booklet).
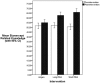
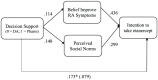
Results: We had 402 RA patients participate in the study (response rate, 52%). Of the patients randomized to the Pharm Booklet, 30.6% elected to initiate etanercept. Only 14.6% and 14.0% of patients who reviewed the LONG DA or SHORT DA choose to take etanercept (χ2 = 15.7; P < 0.001). Patients who reviewed the LONG DA or SHORT DA had a greater increase in knowledge about etanercept than those who reviewed the Pharm Booklet. There was no difference in decisional conflict among the groups. A logistic regression model explained 44.2% (R2 = 0.442) of patient choice to intensify therapy by initiating etanercept. The strongest predictor of choice to intensify therapy were beliefs about etanercept's ability to improve symptoms (OR = 2.56, 96%CI [1.71, 3.80]), and its use by others like the respondent (OR = 2.24, 95%CI [1.49, 3.35]). Mediation analysis confirmed the presence of a partial mediating effect of decision support on patients' intent to take etanercept (OR = 0.59, 95%CI [0.39, 0.89]).
Conclusions: Patients supported by the Pharm Booklet were twice as likely to choose to intensify therapy. The Pharm Booklet's effects are partially mediated through persuasive communication techniques that influence patients' beliefs that symptoms will improve, and increase social normative beliefs, rather than by increasing the relevant knowledge, clarifying patient values about positive or negative treatment outcomes, or increasing their self-efficacy.
Keywords: beliefs about medications; causal mediation; decision making; disease-modifying anti-rheumatic drugs; etanercept; evaluative conditioning; patient decision aid; rheumatoid arthritis.
Figures


References
-
- Feldman-Stewart D, O’Brien MA, Clayman M, et al. Providing Information About Options, 2012. Available from: URL: http://ipdas.ohri.ca/IPDAS-Chapter-B.pdf. - PMC - PubMed
-
- Martin RW, Head AJ, Rene J, et al. Patient decision-making related to antirheumatic drugs in rheumatoid arthritis: the importance of patient trust of physician. J Rheumatol. 2008;35:618–24. - PubMed
-
- Martin RW, Brower ME, Geralds A, Gallagher PJ, Tellinghuisen DJ. An experimental evaluation of patient decision aid design to communicate the effects of medications on the rate of progression of structural joint damage in rheumatoid arthritis. Patient Educ Couns. 2012;86:329–34. - PubMed
-
- Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Eng J Med. 2000;343:1586–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

