Attentional modulation of neuronal variability in circuit models of cortex
- PMID: 28590902
- PMCID: PMC5476447
- DOI: 10.7554/eLife.23978
Attentional modulation of neuronal variability in circuit models of cortex
Abstract
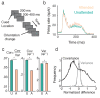
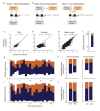
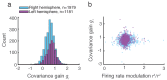
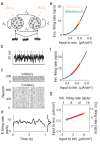
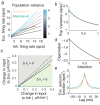
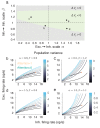
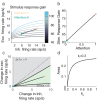
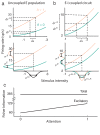
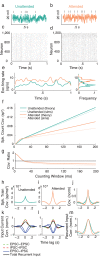
The circuit mechanisms behind shared neural variability (noise correlation) and its dependence on neural state are poorly understood. Visual attention is well-suited to constrain cortical models of response variability because attention both increases firing rates and their stimulus sensitivity, as well as decreases noise correlations. We provide a novel analysis of population recordings in rhesus primate visual area V4 showing that a single biophysical mechanism may underlie these diverse neural correlates of attention. We explore model cortical networks where top-down mediated increases in excitability, distributed across excitatory and inhibitory targets, capture the key neuronal correlates of attention. Our models predict that top-down signals primarily affect inhibitory neurons, whereas excitatory neurons are more sensitive to stimulus specific bottom-up inputs. Accounting for trial variability in models of state dependent modulation of neuronal activity is a critical step in building a mechanistic theory of neuronal cognition.
Keywords: inhibitory feedback; mean field model; neural correlates of attention; neuroscience; noise correlations; rhesus macaque.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
Similar articles
-
Stimulus Dependence of Correlated Variability across Cortical Areas.J Neurosci. 2016 Jul 13;36(28):7546-56. doi: 10.1523/JNEUROSCI.0504-16.2016. J Neurosci. 2016. PMID: 27413163 Free PMC article.
-
Circuit Models of Low-Dimensional Shared Variability in Cortical Networks.Neuron. 2019 Jan 16;101(2):337-348.e4. doi: 10.1016/j.neuron.2018.11.034. Epub 2018 Dec 20. Neuron. 2019. PMID: 30581012 Free PMC article.
-
Stimulus competition by inhibitory interference.Neural Comput. 2005 Nov;17(11):2421-53. doi: 10.1162/0899766054796905. Neural Comput. 2005. PMID: 16156934
-
Neural circuit models for computations in early visual cortex.Curr Opin Neurobiol. 2011 Oct;21(5):808-15. doi: 10.1016/j.conb.2011.07.005. Epub 2011 Aug 26. Curr Opin Neurobiol. 2011. PMID: 21873046 Review.
-
Bottom-up and top-down dynamics in visual cortex.Prog Brain Res. 2005;149:65-81. doi: 10.1016/S0079-6123(05)49006-8. Prog Brain Res. 2005. PMID: 16226577 Review.
Cited by
-
Behavioural stochastic resonance across the lifespan.Cogn Affect Behav Neurosci. 2024 Dec;24(6):1048-1064. doi: 10.3758/s13415-024-01220-w. Epub 2024 Sep 10. Cogn Affect Behav Neurosci. 2024. PMID: 39256251 Free PMC article.
-
Early steps toward understanding neuronal communication.Curr Opin Neurol. 2018 Feb;31(1):59-65. doi: 10.1097/WCO.0000000000000512. Curr Opin Neurol. 2018. PMID: 29076880 Free PMC article. Review.
-
Hippocampal spatial view cells for memory and navigation, and their underlying connectivity in humans.Hippocampus. 2023 May;33(5):533-572. doi: 10.1002/hipo.23467. Epub 2022 Sep 7. Hippocampus. 2023. PMID: 36070199 Free PMC article. Review.
-
Endogenous attention improves perception in amblyopic macaques.J Vis. 2018 Mar 1;18(3):11. doi: 10.1167/18.3.11. J Vis. 2018. PMID: 29677324 Free PMC article.
-
Probing mechanisms of visual spatial attention in mice.Trends Neurosci. 2021 Oct;44(10):822-836. doi: 10.1016/j.tins.2021.07.009. Epub 2021 Aug 23. Trends Neurosci. 2021. PMID: 34446296 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

