Single dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission in Cambodia: An open-label randomized trial
- PMID: 28591198
- PMCID: PMC5462369
- DOI: 10.1371/journal.pone.0168702
Single dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission in Cambodia: An open-label randomized trial
Abstract
Background: Single low dose primaquine (SLD PQ, 0.25mg/kg) is recommended in combination with artemisinin-based combination therapy (ACT) as a gametocytocide to prevent Plasmodium falciparum transmission in areas threatened by artemisinin resistance. To date, no randomized controlled trials have measured primaquine's effect on infectiousness to Anopheline mosquitoes in Southeast Asia.
Methods: Cambodian adults with uncomplicated falciparum malaria were randomized to receive a single 45mg dose of primaquine (equivalent to three SLD PQ) or no primaquine after the third dose of dihydroartemisin-piperaquine (DHP) therapy. A membrane-feeding assay measured infectiousness to Anopheles dirus on days 0, 3, 7, and 14 of blood-stage therapy. Gametocytemia was evaluated by microscopy and reverse-transcriptase PCR.
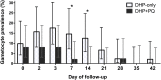
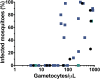
Results: Prior to trial halt for poor DHP treatment efficacy, 101 participants were randomized and 50 received primaquine. Overall microscopic gametocyte prevalence was low (9%), but gametocytemic subjects given primaquine were gametocyte-free by day 14, and significantly less likely to harbor gametocytes by day 7 compared to those treated with DHP-alone, who remained gametocytemic for a median of two weeks. Only one infectious subject was randomized to the primaquine group, precluding assessment of transmission-blocking efficacy. However, he showed a two-fold reduction in oocyst density of infected mosquitoes less than 24 hours after primaquine dosing. In the DHP-alone group, four subjects remained infectious through day 14, infecting roughly the same number of mosquitoes pre and post-treatment. Overall, microscopic gametocytemia was an excellent predictor of infectiousness, and performed better than submicroscopic gametocytemia post-treatment, with none of 474 mosquitoes infected post-treatment arising from submicroscopic gametocytes.
Conclusions: In a setting of established ACT resistance, a single dose of 45mg primaquine added to DHP rapidly and significantly reduced gametocytemia, while DHP-alone failed to reduce gametocytemia and prevent malaria transmission to mosquitoes. Continued efforts to make single dose primaquine widely available are needed to help achieve malaria elimination.
Conflict of interest statement
Figures


References
-
- World Health Organization. Global malaria control and elimination. Report of a meeting on containment of artemisinin tolerance, April 2008. http://www.who.int/malaria/publications/atoz/9789241596817/en/.
-
- Saunders DL, Vanachayangkul P, Lon C, U.S. Army Military Malaria Research Program, National Center for Parasitology, Entomology, and Malaria Control (CNM), Royal Cambodian Armed Forces. Dihydroartemisinin-piperaquine failure in Cambodia. N Engl J Med. 2014;371: 484–485. 10.1056/NEJMc1403007 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

