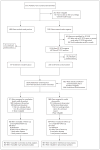
Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma
- PMID: 28591523
- PMCID: PMC5548388
- DOI: 10.1056/NEJMoa1613210
Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma
Abstract
Background: Sentinel-lymph-node biopsy is associated with increased melanoma-specific survival (i.e., survival until death from melanoma) among patients with node-positive intermediate-thickness melanomas (1.2 to 3.5 mm). The value of completion lymph-node dissection for patients with sentinel-node metastases is not clear.
Methods: In an international trial, we randomly assigned patients with sentinel-node metastases detected by means of standard pathological assessment or a multimarker molecular assay to immediate completion lymph-node dissection (dissection group) or nodal observation with ultrasonography (observation group). The primary end point was melanoma-specific survival. Secondary end points included disease-free survival and the cumulative rate of nonsentinel-node metastasis.
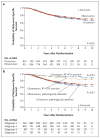
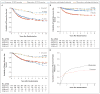
Results: Immediate completion lymph-node dissection was not associated with increased melanoma-specific survival among 1934 patients with data that could be evaluated in an intention-to-treat analysis or among 1755 patients in the per-protocol analysis. In the per-protocol analysis, the mean (±SE) 3-year rate of melanoma-specific survival was similar in the dissection group and the observation group (86±1.3% and 86±1.2%, respectively; P=0.42 by the log-rank test) at a median follow-up of 43 months. The rate of disease-free survival was slightly higher in the dissection group than in the observation group (68±1.7% and 63±1.7%, respectively; P=0.05 by the log-rank test) at 3 years, based on an increased rate of disease control in the regional nodes at 3 years (92±1.0% vs. 77±1.5%; P<0.001 by the log-rank test); these results must be interpreted with caution. Nonsentinel-node metastases, identified in 11.5% of the patients in the dissection group, were a strong, independent prognostic factor for recurrence (hazard ratio, 1.78; P=0.005). Lymphedema was observed in 24.1% of the patients in the dissection group and in 6.3% of those in the observation group.
Conclusions: Immediate completion lymph-node dissection increased the rate of regional disease control and provided prognostic information but did not increase melanoma-specific survival among patients with melanoma and sentinel-node metastases. (Funded by the National Cancer Institute and others; MSLT-II ClinicalTrials.gov number, NCT00297895 .).
Figures
Comment in
-
The Enigma of Regional Lymph Nodes in Melanoma.N Engl J Med. 2017 Jun 8;376(23):2280-2281. doi: 10.1056/NEJMe1704290. N Engl J Med. 2017. PMID: 28591534 No abstract available.
-
Completion dissection or observation for sentinel-node metastasis in melanoma.Dermatol Ther. 2017 Nov;30(6). doi: 10.1111/dth.12544. Epub 2017 Aug 24. Dermatol Ther. 2017. PMID: 28836714 No abstract available.
-
Melanoma Sentinel-Node Metastasis.N Engl J Med. 2017 Aug 31;377(9):891-2. doi: 10.1056/NEJMc1708918. N Engl J Med. 2017. PMID: 28854099 No abstract available.
-
Melanoma Sentinel-Node Metastasis.N Engl J Med. 2017 Aug 31;377(9):891. doi: 10.1056/NEJMc1708918. N Engl J Med. 2017. PMID: 28858461 No abstract available.
-
[Survival after radical completion dissection or observation for sentinel node metastasis in melanoma].Strahlenther Onkol. 2017 Nov;193(11):989-990. doi: 10.1007/s00066-017-1200-3. Strahlenther Onkol. 2017. PMID: 28871405 German. No abstract available.
References
-
- Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17. - PubMed
-
- Australian Cancer Network Melanoma Guidelines Revision Working Party. Clinical practice guidelines for the management of melanoma in Australia and New Zealand. Wellington, New Zealand: Cancer Council Australia/Australian Cancer Network; Oct, 2008.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
