Sonic Hedgehog Activates Phospholipase A2 to Enhance Smoothened Ciliary Translocation
- PMID: 28591579
- PMCID: PMC5520665
- DOI: 10.1016/j.celrep.2017.05.033
Sonic Hedgehog Activates Phospholipase A2 to Enhance Smoothened Ciliary Translocation
Abstract
The G protein-coupled receptor Smoothened (Smo) is the signal transducer of the Sonic Hedgehog (Shh) pathway. Smo signals through G protein-dependent and -independent routes, with G protein-independent canonical signaling to Gli effectors requiring Smo accumulation in the primary cilium. The mechanisms controlling Smo activation and trafficking are not yet clear but likely entail small-molecule binding to pockets in its extracellular cysteine-rich domain (CRD) and/or transmembrane bundle. Here, we demonstrate that the cytosolic phospholipase cPLA2α is activated through Gβγ downstream of Smo to release arachidonic acid. Arachidonic acid binds Smo and synergizes with CRD-binding agonists, promoting Smo ciliary trafficking and high-level signaling. Chemical or genetic cPLA2α inhibition dampens Smo signaling to Gli, revealing an unexpected contribution of G protein-dependent signaling to canonical pathway activity. Arachidonic acid displaces the Smo transmembrane domain inhibitor cyclopamine to rescue CRD agonist-induced signaling, suggesting that arachidonic acid may target the transmembrane bundle to allosterically enhance signaling by CRD agonist-bound Smo.
Keywords: Smoothened; Sonic Hedgehog; arachidonic acid; phospholipase A2; primary cilium; signal transduction.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

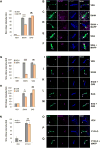
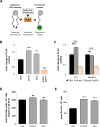
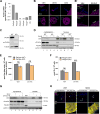
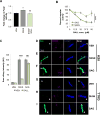
References
-
- Balsinde J, Winstead MV, Dennis EA. Phospholipase A(2) regulation of arachidonic acid mobilization. FEBS Lett. 2002;531:2–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

