A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-culture-Specific Expression Profile and Inflammatory Response
- PMID: 28591653
- PMCID: PMC5470330
- DOI: 10.1016/j.stemcr.2017.05.017
A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-culture-Specific Expression Profile and Inflammatory Response
Abstract
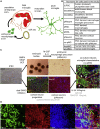
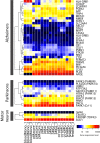
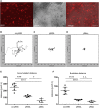
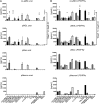
Microglia are increasingly implicated in brain pathology, particularly neurodegenerative disease, with many genes implicated in Alzheimer's, Parkinson's, and motor neuron disease expressed in microglia. There is, therefore, a need for authentic, efficient in vitro models to study human microglial pathological mechanisms. Microglia originate from the yolk sac as MYB-independent macrophages, migrating into the developing brain to complete differentiation. Here, we recapitulate microglial ontogeny by highly efficient differentiation of embryonic MYB-independent iPSC-derived macrophages then co-culture them with iPSC-derived cortical neurons. Co-cultures retain neuronal maturity and functionality for many weeks. Co-culture microglia express key microglia-specific markers and neurodegenerative disease-relevant genes, develop highly dynamic ramifications, and are phagocytic. Upon activation they become more ameboid, releasing multiple microglia-relevant cytokines. Importantly, co-culture microglia downregulate pathogen-response pathways, upregulate homeostatic function pathways, and promote a more anti-inflammatory and pro-remodeling cytokine response than corresponding monocultures, demonstrating that co-cultures are preferable for modeling authentic microglial physiology.
Keywords: Alzheimer's disease; Parkinson's disease; cortical neurons; human; iPSC; induced pluripotent stem cell; macrophage; microglia; neurodegeneration; neuroinflammation.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

