Clinical evaluation of integrated panel testing by next-generation sequencing for somatic mutations in neuroblastomas with MYCN unamplification
- PMID: 28591696
- PMCID: PMC5564799
- DOI: 10.18632/oncotarget.17917
Clinical evaluation of integrated panel testing by next-generation sequencing for somatic mutations in neuroblastomas with MYCN unamplification
Abstract
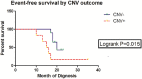
Neuroblastomas (NBs) exhibit heterogeneity and show clinically significant prognosis classified by genetic alterations. Among prognostic genes or genome factors, MYCN amplification (MNA) is the most established genomic marker of poor prognosis in patients with NB. However, the prognostic classification of more than 60% of patients without MNA has yet to be clarified. In this study, the application of target next-generation sequencing (NGS) was extended on the basis of a comprehensive panel of regions where copy number variations (CNVs) or point mutations occurred to improve the prognostic evaluation of these patients and obtain the sequence of 33 patients without MNA. A mean coverage depth of 887× was determined in the target regions in all of the samples, and the mapped read percentage was more than 99%. Somatic mutations in patients without MNA could be precisely defined on the basis of these findings, and 17 unique somatic aberrations, including 14 genes, were identified in 11 patients. Among these variations, most were CNVs with a number of 13. The 3-year event-free survival (EFS) of CNV(-) patients was 60.0% compared with the EFS (16.7%) of CNV(+) patients (P = 0.015, HR = 0.1344, 95%, CI = 0.027 to 0.678). CNVs were also associated with unfavorable histological characteristics (P = 0.003) and likely to occur in stage 4 (P = 0.041). These results might further indicate the role of CNVs in NB chemotherapy resistance (P = 0.059) and show CNVs as a therapeutic target. In multivariate analysis, the presence of CNVs was a clinically negative prognostic marker that impaired the outcome of patients without MNA and associated with poor prognosis in this tumor subset. Comprehensive genetic/genomic profiling instead of focusing on single genetic marker should be performed through in-depth NGS that could reveal prognostic information, improve NB target therapy, and provide a basis for investigations on NB pathogenesis.
Keywords: DNA copy number variations; high-throughput nucleotide sequencing; neuroblastoma; prognosis; treatment.
Conflict of interest statement
The authors declared no potential financial conflicts of interest.
Figures





Similar articles
-
BLM germline and somatic PKMYT1 and AHCY mutations: Genetic variations beyond MYCN and prognosis in neuroblastoma.Med Hypotheses. 2016 Dec;97:22-25. doi: 10.1016/j.mehy.2016.10.008. Epub 2016 Oct 20. Med Hypotheses. 2016. PMID: 27876123
-
Genomic Amplifications and Distal 6q Loss: Novel Markers for Poor Survival in High-risk Neuroblastoma Patients.J Natl Cancer Inst. 2018 Oct 1;110(10):1084-1093. doi: 10.1093/jnci/djy022. J Natl Cancer Inst. 2018. PMID: 29514301 Free PMC article.
-
Co-expression analysis identifies long noncoding RNA SNHG1 as a novel predictor for event-free survival in neuroblastoma.Oncotarget. 2016 Sep 6;7(36):58022-58037. doi: 10.18632/oncotarget.11158. Oncotarget. 2016. PMID: 27517149 Free PMC article.
-
Diagnostic accuracy of circulating-free DNA for the determination of MYCN amplification status in advanced-stage neuroblastoma: a systematic review and meta-analysis.Br J Cancer. 2020 Mar;122(7):1077-1084. doi: 10.1038/s41416-020-0740-y. Epub 2020 Feb 4. Br J Cancer. 2020. PMID: 32015512 Free PMC article.
-
Towards diagnostic application of non-coding RNAs in neuroblastoma.Expert Rev Mol Diagn. 2016 Dec;16(12):1307-1313. doi: 10.1080/14737159.2016.1256207. Epub 2016 Nov 15. Expert Rev Mol Diagn. 2016. PMID: 27813435 Review.
Cited by
-
Long noncoding RNA LINC01296 promotes tumor growth and progression by sponging miR-5095 in human cholangiocarcinoma.Int J Oncol. 2018 Jun;52(6):1777-1786. doi: 10.3892/ijo.2018.4362. Epub 2018 Apr 5. Int J Oncol. 2018. PMID: 29620172 Free PMC article.
-
Neuroblastoma Risk Assessment and Treatment Stratification with Hybrid Capture-Based Panel Sequencing.J Pers Med. 2021 Jul 22;11(8):691. doi: 10.3390/jpm11080691. J Pers Med. 2021. PMID: 34442335 Free PMC article.
-
Identification of ALK Mutation in Neuroblastoma on the Point of Molecular Heterogeneity.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338231211138. doi: 10.1177/15330338231211138. Technol Cancer Res Treat. 2023. PMID: 37964559 Free PMC article.
References
-
- London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H, Thorner P, Brodeur G, Maris JM, Reynolds CP, Cohn SL. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. Journal of clinical oncology. 2005;23:6459–6465. - PubMed
-
- Shimada H, Chatten J, Newton WA, Jr, Sachs N, Hamoudi AB, Chiba T, Marsden HB, Misugi K. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. Journal of the National Cancer Institute. 1984;73:405–416. - PubMed
-
- Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. The New England journal of medicine. 1985;313:1111–1116. - PubMed
-
- Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86:349–363. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

