BRSK2 induced by nutrient deprivation promotes Akt activity in pancreatic cancer via downregulation of mTOR activity
- PMID: 28591720
- PMCID: PMC5546509
- DOI: 10.18632/oncotarget.17965
BRSK2 induced by nutrient deprivation promotes Akt activity in pancreatic cancer via downregulation of mTOR activity
Abstract
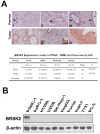
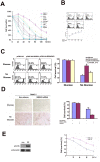
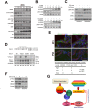
Neoplastic cells in pancreatic ductual adenocarcinoma (PDAC) survive in an energy-deprived milieu, and hyper-activation of Akt is thought to contribute to the neoplastic cell survival in PDAC. Kras activating mutations, common in PDAC, was believed to be the major driver of Akt activation. However, the inhibitor to Kras was not therapeutic for PDAC patients. This implied that PDAC cells might harbor an intrinsic merit that strengthens Akt activity. Here we showed that BRSK2, a serine/threonine-protein kinase of AMPK family, was induced by nutrient deprivation in PDAC cells and suppressed mTORC1 activity via phosphorylation of tuberous sclerosis complex 2 (TSC2). The suppression of mTORC1 activity in PDAC results in a dominant loss of feedback inhibition on Akt activity by mTORC1, consequently enhancing cell survival. This finding indicates that the intrinsic molecular merit that BRSK2 provides is a survival advantage to PDAC cells and strengthens the invasiveness of these neoplastic cells in energy-deprived environments.
Keywords: AMPK; Akt; BRSK2; PDAC; mTOR.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


Similar articles
-
Ribonucleoprotein HNRNPA2B1 interacts with and regulates oncogenic KRAS in pancreatic ductal adenocarcinoma cells.Gastroenterology. 2014 Oct;147(4):882-892.e8. doi: 10.1053/j.gastro.2014.06.041. Epub 2014 Jul 3. Gastroenterology. 2014. PMID: 24998203
-
Different patterns of Akt and ERK feedback activation in response to rapamycin, active-site mTOR inhibitors and metformin in pancreatic cancer cells.PLoS One. 2013;8(2):e57289. doi: 10.1371/journal.pone.0057289. Epub 2013 Feb 21. PLoS One. 2013. PMID: 23437362 Free PMC article.
-
The suppressive efficacy of THZ1 depends on KRAS mutation subtype and is associated with super-enhancer activity and the PI3K/AKT/mTOR signalling in pancreatic ductal adenocarcinoma: A hypothesis-generating study.Clin Transl Med. 2023 Dec;13(12):e1500. doi: 10.1002/ctm2.1500. Clin Transl Med. 2023. PMID: 38037549 Free PMC article.
-
Prospects of targeting PI3K/AKT/mTOR pathway in pancreatic cancer.Crit Rev Oncol Hematol. 2022 Aug;176:103749. doi: 10.1016/j.critrevonc.2022.103749. Epub 2022 Jun 18. Crit Rev Oncol Hematol. 2022. PMID: 35728737 Review.
-
RES-529: a PI3K/AKT/mTOR pathway inhibitor that dissociates the mTORC1 and mTORC2 complexes.Anticancer Drugs. 2016 Jul;27(6):475-87. doi: 10.1097/CAD.0000000000000354. Anticancer Drugs. 2016. PMID: 26918392 Free PMC article. Review.
Cited by
-
Gain-of-function genetic screen of the kinome reveals BRSK2 as an inhibitor of the NRF2 transcription factor.J Cell Sci. 2020 Jul 15;133(14):jcs241356. doi: 10.1242/jcs.241356. J Cell Sci. 2020. PMID: 32546533 Free PMC article.
-
A novel machine learning model based on ubiquitin-related gene pairs and clinical features to predict prognosis and treatment effect in colon adenocarcinoma.Eur J Med Res. 2023 Jan 21;28(1):41. doi: 10.1186/s40001-023-00993-z. Eur J Med Res. 2023. PMID: 36681855 Free PMC article.
-
Construction of a pancreatic cancer nerve invasion system using brain and pancreatic cancer organoids.J Tissue Eng. 2023 Jan 6;14:20417314221147113. doi: 10.1177/20417314221147113. eCollection 2023 Jan-Dec. J Tissue Eng. 2023. PMID: 36636100 Free PMC article.
-
Pan-Cancer Analysis of Human Kinome Gene Expression and Promoter DNA Methylation Identifies Dark Kinase Biomarkers in Multiple Cancers.Cancers (Basel). 2021 Mar 10;13(6):1189. doi: 10.3390/cancers13061189. Cancers (Basel). 2021. PMID: 33801837 Free PMC article.
-
DNA methylation patterns in bladder tumors of African American patients point to distinct alterations in xenobiotic metabolism.Carcinogenesis. 2019 Nov 25;40(11):1332-1340. doi: 10.1093/carcin/bgz128. Carcinogenesis. 2019. PMID: 31284295 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

