Sustained Immunogenicity of 2-dose Human Papillomavirus 16/18 AS04-adjuvanted Vaccine Schedules in Girls Aged 9-14 Years: A Randomized Trial
- PMID: 28591778
- PMCID: PMC5853959
- DOI: 10.1093/infdis/jix154
Sustained Immunogenicity of 2-dose Human Papillomavirus 16/18 AS04-adjuvanted Vaccine Schedules in Girls Aged 9-14 Years: A Randomized Trial
Abstract
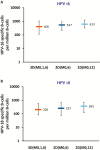
Background: We previously reported the noninferiority 1 month after the last dose of 2-dose human papillomavirus 16/18 AS04-adjuvanted (AS04-HPV-16/18) vaccine schedules at months 0 and 6 (2D_M0,6) and months 0 and 12 (2D_M0,12) in girls aged 9-14 years compared with a 3-dose schedule at months 0, 1, and 6 (3D_M0,1,6) in women aged 15-25 years. Here, we report the results at study end (month 36 [M36]).
Methods: Girls were randomized 1:1 and received 2 vaccine doses either 6 months (2D_M0,6) or 12 months apart (2D_M0,12); women received 3 doses at months 0, 1, and 6 (3D_M0,1,6). Endpoints included noninferiority of HPV-16/18 antibodies for 2D_M0,6 versus 3D_M0,1,6; 2D_M0,12 versus 3D_M0,1,6; and 2D_M0,12 versus 2D_M0,6; and assessment of neutralizing antibodies, T cells, B cells, and safety.
Results: At M36, the 2D_M0,6 and 2D_M0,12 schedules remained noninferior to the 3D_M0,1,6 schedule in terms of seroconversion rates and 3D/2D geometric mean titers for anti-HPV-16 and anti-HPV-18. All schedules elicited sustained immune responses up to M36.
Conclusions: Both 2-dose schedules in young girls remained noninferior to the 3-dose schedule in women up to study conclusion at M36. The AS04-HPV-16/18 vaccine administered as a 2-dose schedule was immunogenic and well tolerated in young girls.
Keywords: 2-dose schedule; Cervarix; cervical cancer; human papillomavirus (HPV).
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures




Comment in
-
Reply to Poddighe.J Infect Dis. 2017 Sep 15;216(6):783-785. doi: 10.1093/infdis/jix365. J Infect Dis. 2017. PMID: 28934440 Free PMC article. No abstract available.
-
2-Dose Schedule of AS04-Adjuvanted Human Papillomavirus Types 16/18 Vaccine.J Infect Dis. 2017 Sep 15;216(6):782-783. doi: 10.1093/infdis/jix364. J Infect Dis. 2017. PMID: 28934441 No abstract available.
References
-
- ICO Information Centre on HPV and Cancer. Human Papillomavirus and Related Diseases Report–World. http://hpvcentre.net/statistics/reports/XWX.pdf. Accessed April 10 2016.
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. - PubMed
-
- Bosch FX, Burchell AN, Schiffman M et al. . Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008; 26Suppl 10:K1–16. - PubMed
-
- de Sanjose S, Quint WG, Alemany L et al. ; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–56. - PubMed
-
- Muñoz N, Bosch FX, de Sanjosé S et al. ; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

