Next generation human skin constructs as advanced tools for drug development
- PMID: 28592171
- PMCID: PMC5786367
- DOI: 10.1177/1535370217712690
Next generation human skin constructs as advanced tools for drug development
Abstract
Many diseases, as well as side effects of drugs, manifest themselves through skin symptoms. Skin is a complex tissue that hosts various specialized cell types and performs many roles including physical barrier, immune and sensory functions. Therefore, modeling skin in vitro presents technical challenges for tissue engineering. Since the first attempts at engineering human epidermis in 1970s, there has been a growing interest in generating full-thickness skin constructs mimicking physiological functions by incorporating various skin components, such as vasculature and melanocytes for pigmentation. Development of biomimetic in vitro human skin models with these physiological functions provides a new tool for drug discovery, disease modeling, regenerative medicine and basic research for skin biology. This goal, however, has long been delayed by the limited availability of different cell types, the challenges in establishing co-culture conditions, and the ability to recapitulate the 3D anatomy of the skin. Recent breakthroughs in induced pluripotent stem cell (iPSC) technology and microfabrication techniques such as 3D-printing have allowed for building more reliable and complex in vitro skin models for pharmaceutical screening. In this review, we focus on the current developments and prevailing challenges in generating skin constructs with vasculature, skin appendages such as hair follicles, pigmentation, immune response, innervation, and hypodermis. Furthermore, we discuss the promising advances that iPSC technology offers in order to generate in vitro models of genetic skin diseases, such as epidermolysis bullosa and psoriasis. We also discuss how future integration of the next generation human skin constructs onto microfluidic platforms along with other tissues could revolutionize the early stages of drug development by creating reliable evaluation of patient-specific effects of pharmaceutical agents. Impact statement Skin is a complex tissue that hosts various specialized cell types and performs many roles including barrier, immune, and sensory functions. For human-relevant drug testing, there has been a growing interest in building more physiological skin constructs by incorporating different skin components, such as vasculature, appendages, pigment, innervation, and adipose tissue. This paper provides an overview of the strategies to build complex human skin constructs that can faithfully recapitulate human skin and thus can be used in drug development targeting skin diseases. In particular, we discuss recent developments and remaining challenges in incorporating various skin components, availability of iPSC-derived skin cell types and in vitro skin disease models. In addition, we provide insights on the future integration of these complex skin models with other organs on microfluidic platforms as well as potential readout technologies for high-throughput drug screening.
Keywords: Skin constructs; drug testing; microphysiological systems; skin-on-a-chip.
Figures
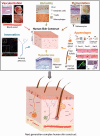
References
-
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell 2004; 116: 769–78. - PubMed
-
- Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol 2002; 118: 216–25. - PubMed
-
- Botchkarev VA, Paus R. Molecular biology of hair morphogenesis: development and cycling. J Exp Zool B Mol Dev Evol 2003; 298: 164–80. - PubMed
-
- Natarajan VT, Ganju P, Ramkumar A, Grover R, Gokhale RS. Multifaceted pathways protect human skin from UV radiation. Nat Chem Biol 2014; 10: 542–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

