Global gene expression in muscle from fasted/refed trout reveals up-regulation of genes promoting myofibre hypertrophy but not myofibre production
- PMID: 28592307
- PMCID: PMC5463356
- DOI: 10.1186/s12864-017-3837-9
Global gene expression in muscle from fasted/refed trout reveals up-regulation of genes promoting myofibre hypertrophy but not myofibre production
Abstract
Background: Compensatory growth is a phase of rapid growth, greater than the growth rate of control animals, that occurs after a period of growth-stunting conditions. Fish show a capacity for compensatory growth after alleviation of dietary restriction, but the underlying cellular mechanisms are unknown. To learn more about the contribution of genes regulating hypertrophy (an increase in muscle fibre size) and hyperplasia (the generation of new muscle fibres) in the compensatory muscle growth response in fish, we used high-density microarray analysis to investigate the global gene expression in muscle of trout during a fasting-refeeding schedule and in muscle of control-fed trout displaying normal growth.
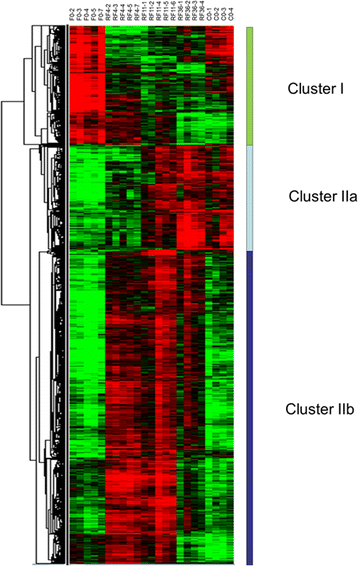
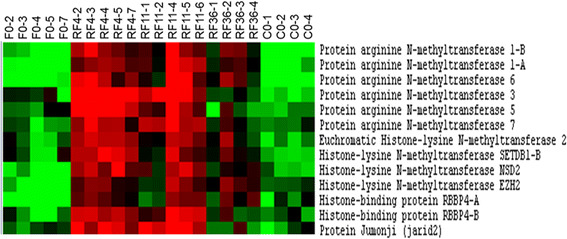
Results: The compensatory muscle growth signature, as defined by genes up-regulated in muscles of refed trout compared with control-fed trout, showed enrichment in functional categories related to protein biosynthesis and maturation, such as RNA processing, ribonucleoprotein complex biogenesis, ribosome biogenesis, translation and protein folding. This signature was also enriched in chromatin-remodelling factors of the protein arginine N-methyl transferase family. Unexpectedly, functional categories related to cell division and DNA replication were not inferred from the molecular signature of compensatory muscle growth, and this signature contained virtually none of the genes previously reported to be up-regulated in hyperplastic growth zones of the late trout embryo myotome and to potentially be involved in production of new myofibres, notably genes encoding myogenic regulatory factors, transmembrane receptors essential for myoblast fusion or myofibrillar proteins predominant in nascent myofibres.
Conclusion: Genes promoting myofibre growth, but not myofibre formation, were up-regulated in muscles of refed trout compared with continually fed trout. This suggests that a compensatory muscle growth response, resulting from the stimulation of hypertrophy but not the stimulation of hyperplasia, occurs in trout after refeeding. The generation of a large set of genes up-regulated in muscle of refed trout may yield insights into the molecular and cellular mechanisms controlling skeletal muscle mass in teleost and serve as a useful list of potential molecular markers of muscle growth in fish.
Keywords: Gene expression; Muscle growth; Muscle hyperplasia; Muscle hypertrophy; Teleost; Transcriptome.
Figures


Similar articles
-
Gene expression profiling of trout regenerating muscle reveals common transcriptional signatures with hyperplastic growth zones of the post-embryonic myotome.BMC Genomics. 2016 Oct 18;17(1):810. doi: 10.1186/s12864-016-3160-x. BMC Genomics. 2016. PMID: 27756225 Free PMC article.
-
Histological, transcriptomic and in vitro analysis reveal an intrinsic activated state of myogenic precursors in hyperplasic muscle of trout.BMC Genomics. 2018 Dec 3;19(1):865. doi: 10.1186/s12864-018-5248-y. BMC Genomics. 2018. PMID: 30509177 Free PMC article.
-
Dynamic gene expression in fish muscle during recovery growth induced by a fasting-refeeding schedule.BMC Genomics. 2007 Nov 28;8:438. doi: 10.1186/1471-2164-8-438. BMC Genomics. 2007. PMID: 18045468 Free PMC article.
-
Exercise-induced skeletal muscle growth. Hypertrophy or hyperplasia?Sports Med. 1986 May-Jun;3(3):190-200. doi: 10.2165/00007256-198603030-00003. Sports Med. 1986. PMID: 3520748 Review.
-
Paradigms of growth in fish.Comp Biochem Physiol B Biochem Mol Biol. 2001 Jun;129(2-3):207-19. doi: 10.1016/s1096-4959(01)00312-8. Comp Biochem Physiol B Biochem Mol Biol. 2001. PMID: 11399452 Review.
Cited by
-
Effect of fasting and subsequent refeeding on the transcriptional profiles of brain in juvenile Spinibarbus hollandi.PLoS One. 2019 Mar 28;14(3):e0214589. doi: 10.1371/journal.pone.0214589. eCollection 2019. PLoS One. 2019. PMID: 30921420 Free PMC article.
-
Aberrant mitochondrial homeostasis at the crossroad of musculoskeletal ageing and non-small cell lung cancer.PLoS One. 2022 Sep 6;17(9):e0273766. doi: 10.1371/journal.pone.0273766. eCollection 2022. PLoS One. 2022. PMID: 36067173 Free PMC article.
-
Unravelling the changes during induced vitellogenesis in female European eel through RNA-Seq: What happens to the liver?PLoS One. 2020 Aug 13;15(8):e0236438. doi: 10.1371/journal.pone.0236438. eCollection 2020. PLoS One. 2020. PMID: 32790680 Free PMC article.
-
Gene Expression Profile Provides Novel Insights of Fasting-Refeeding Response in Zebrafish Skeletal Muscle.Nutrients. 2022 May 27;14(11):2239. doi: 10.3390/nu14112239. Nutrients. 2022. PMID: 35684038 Free PMC article.
-
Comparison of Migratory and Resident Populations of Brown Trout Reveals Candidate Genes for Migration Tendency.Genome Biol Evol. 2018 Jun 1;10(6):1493-1503. doi: 10.1093/gbe/evy102. Genome Biol Evol. 2018. PMID: 29850813 Free PMC article.
References
-
- Rowlerson A, Veggetti A. Cellular mechanisms of post-embryonic muscle growth in aquaculture species. In: Johnston IA, editor. Muscle development and growth, fish physiology series. San Diego: Academic; 2001. pp. 103–140.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

