Impact of community-initiated Kangaroo Mother Care on survival of low birth weight infants: study protocol for a randomized controlled trial
- PMID: 28592313
- PMCID: PMC5463407
- DOI: 10.1186/s13063-017-1991-7
Impact of community-initiated Kangaroo Mother Care on survival of low birth weight infants: study protocol for a randomized controlled trial
Abstract
Background: Around 70% neonatal deaths occur in low birth weight (LBW) babies. Globally, 15% of babies are born with LBW. Kangaroo Mother Care (KMC) appears to be an effective way to reduce mortality and morbidity among LBW babies. KMC comprises of early and continuous skin-to-skin contact between mother and baby as well as exclusive breastfeeding. Evidence derived from hospital-based studies shows that KMC results in a 40% relative reduction in mortality, a 58% relative reduction in the risk of nosocomial infections or sepsis, shorter hospital stay, and a lower risk of lower respiratory tract infections in babies with birth weight <2000 g. There has been considerable interest in KMC initiated outside health facilities for LBW babies born at home or discharged early. Currently, there is insufficient evidence to support initiation of KMC in the community (cKMC). Formative research in our study setting, where 24% of babies are born with LBW, demonstrated that KMC is feasible and acceptable when initiated at home for LBW babies. The aim of this trial is to determine the impact of cKMC on the survival of these babies.
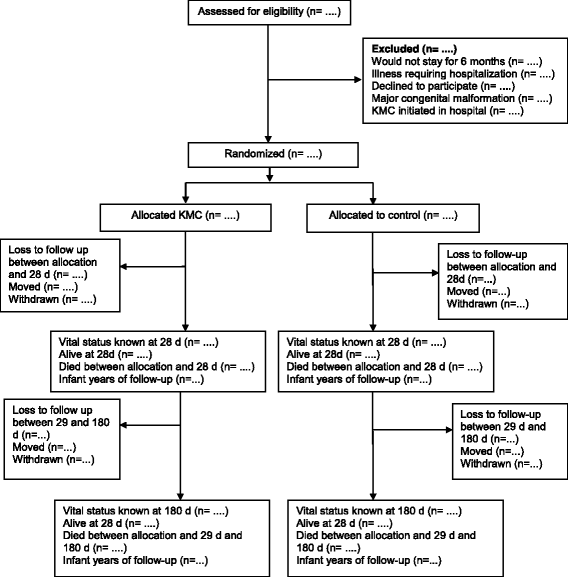
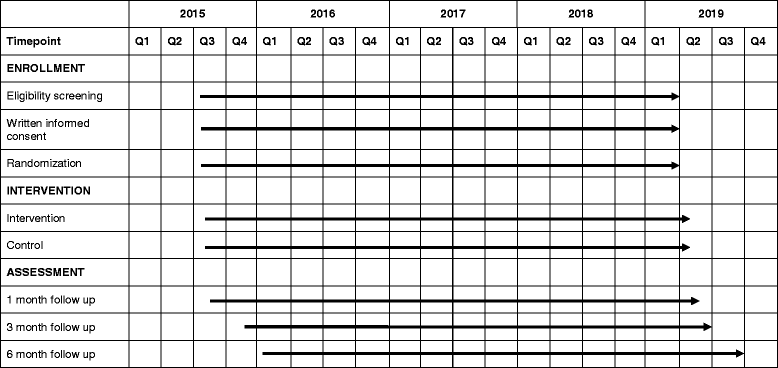
Methods/design: This randomized controlled trial is being undertaken in the Palwal and Faridabad districts in the State of Haryana, India. Neonates weighing 1500-2250 g identified within 3 days of birth and their mothers are being enrolled. Other inclusion criteria are that the family is likely to be available in the study area over the next 6 months, that KMC was not initiated in the delivery facility, and that the infant does not have an illness requiring hospitalization. Eligible neonates are randomized into intervention and control groups. The intervention is delivered through home visits during the first month of life by study workers with a background and education similar to that of workers in the government health system. An independent study team collects mortality and morbidity data as well as anthropometric measurements during periodic home visits. The primary outcomes of the study are postenrollment neonatal mortality and mortality between enrollment and 6 months of age. The secondary outcomes are breastfeeding practices; prevalence of illnesses and care-seeking practices for the same; hospitalizations; weight and length gain; and, in a subsample, neurodevelopment.
Discussion: This efficacy trial will answer the question whether the benefits of KMC observed in hospital settings can also be observed when KMC is started in the community. The formative research used for intervention development suggests that the necessary high level of KMC adoption can be reached in the community, addressing a problem that seriously constrained conclusions in the only other trial in which researchers examined the benefits of cKMC.
Trial registration: ClinicalTrials.gov identifier: NCT02653534 . Registered on 26 December 2015 (retrospectively registered).
Keywords: Community-initiated Kangaroo Mother Care; Low birth weight babies; Mortality.
Figures
References
-
- World Health Organization (WHO). Care of the preterm and/or low-birth-weight newborn. http://www.who.int/maternal_child_adolescent/topics/newborn/care_of_pret.... Accessed 2 Sept 2016.
-
- Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1:e26–36. doi: 10.1016/S2214-109X(13)70006-8. - DOI - PMC - PubMed
-
- Mazumder S, Taneja S, Bhatia K, Yoshida S, Kaur J, Dube B, et al. Efficacy of early neonatal supplementation with vitamin A to reduce mortality in infancy in Haryana, India (Neovita): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:1333–42. doi: 10.1016/S0140-6736(14)60891-6. - DOI - PubMed
-
- World Health Organization (WHO). Preterm birth. Fact sheet no. 363. http://www.who.int/mediacentre/factsheets/fs363/en/. Accessed 2 Sept 2016.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical