State of play and clinical prospects of antibody gene transfer
- PMID: 28592330
- PMCID: PMC5463339
- DOI: 10.1186/s12967-017-1234-4
State of play and clinical prospects of antibody gene transfer
Abstract
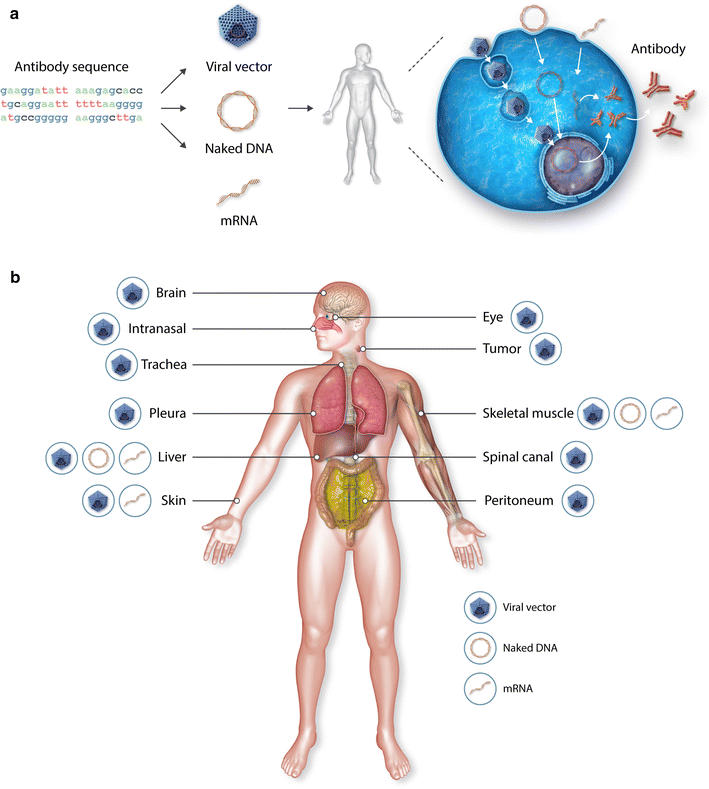
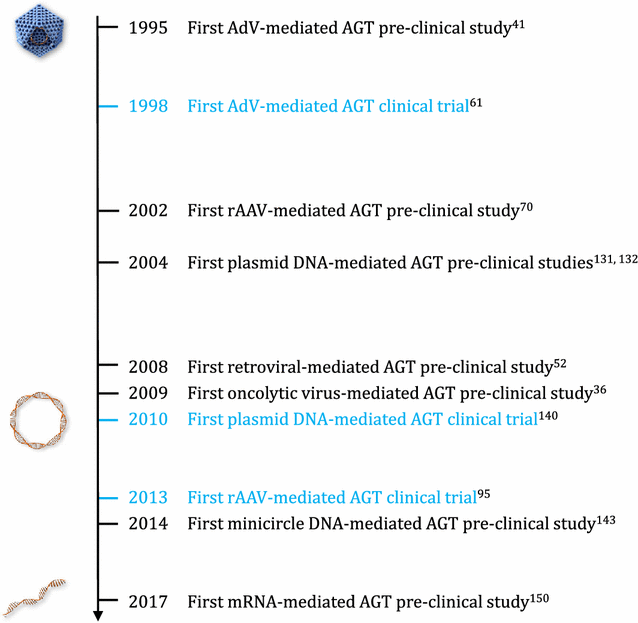
Recombinant monoclonal antibodies (mAbs) are one of today's most successful therapeutic classes in inflammatory diseases and oncology. A wider accessibility and implementation, however, is hampered by the high product cost and prolonged need for frequent administration. The surge in more effective mAb combination therapies further adds to the costs and risk of toxicity. To address these issues, antibody gene transfer seeks to administer to patients the mAb-encoding nucleotide sequence, rather than the mAb protein. This allows the body to produce its own medicine in a cost- and labor-effective manner, for a prolonged period of time. Expressed mAbs can be secreted systemically or locally, depending on the production site. The current review outlines the state of play and clinical prospects of antibody gene transfer, thereby highlighting recent innovations, opportunities and remaining hurdles. Different expression platforms and a multitude of administration sites have been pursued. Viral vector-mediated mAb expression thereby made the most significant strides. Therapeutic proof of concept has been demonstrated in mice and non-human primates, and intramuscular vectored mAb therapy is under clinical evaluation. However, viral vectors face limitations, particularly in terms of immunogenicity. In recent years, naked DNA has gained ground as an alternative. Attained serum mAb titers in mice, however, remain far below those obtained with viral vectors, and robust pharmacokinetic data in larger animals is limited. The broad translatability of DNA-based antibody therapy remains uncertain, despite ongoing evaluation in patients. RNA presents another emerging platform for antibody gene transfer. Early reports in mice show that mRNA may be able to rival with viral vectors in terms of generated serum mAb titers, although expression appears more short-lived. Overall, substantial progress has been made in the clinical translation of antibody gene transfer. While challenges persist, clinical prospects are amplified by ongoing innovations and the versatility of antibody gene transfer. Clinical introduction can be expedited by selecting the platform approach currently best suited for the mAb or disease of interest. Innovations in expression platform, administration and antibody technology are expected to further improve overall safety and efficacy, and unlock the vast clinical potential of antibody gene transfer.
Figures
References
-
- Gils A, Bertolotto A, Mulleman D, Bejan-Angoulvant T, Declerck PJ. Biopharmaceuticals: reference products and biosimilars to treat inflammatory diseases. Ther Drug Monit. 2017 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

