A Short History and Description of Drosophila melanogaster Classical Genetics: Chromosome Aberrations, Forward Genetic Screens, and the Nature of Mutations
- PMID: 28592503
- PMCID: PMC5499179
- DOI: 10.1534/genetics.117.199950
A Short History and Description of Drosophila melanogaster Classical Genetics: Chromosome Aberrations, Forward Genetic Screens, and the Nature of Mutations
Abstract
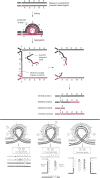
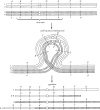
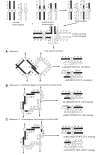
The purpose of this chapter in FlyBook is to acquaint the reader with the Drosophila genome and the ways in which it can be altered by mutation. Much of what follows will be familiar to the experienced Fly Pusher but hopefully will be useful to those just entering the field and are thus unfamiliar with the genome, the history of how it has been and can be altered, and the consequences of those alterations. I will begin with the structure, content, and organization of the genome, followed by the kinds of structural alterations (karyotypic aberrations), how they affect the behavior of chromosomes in meiotic cell division, and how that behavior can be used. Finally, screens for mutations as they have been performed will be discussed. There are several excellent sources of detailed information on Drosophila husbandry and screening that are recommended for those interested in further expanding their familiarity with Drosophila as a research tool and model organism. These are a book by Ralph Greenspan and a review article by John Roote and Andreas Prokop, which should be required reading for any new student entering a fly lab for the first time.
Keywords: FlyBook; balancers; chromosome aberrations; genome; screens.
Copyright © 2017 by the Genetics Society of America.
Figures
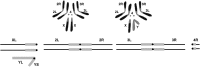











References
-
- Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., et al. , 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195. - PubMed
-
- Ashburner M., Golic K. G., Hawley R. S., 2005. Drosophila: A Laboratory Handbook, Ed. 2 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. pp. 1408.
-
- Auerbach C., Robson J. M., 1946. Chemical production of mutations. Nature 157: 302. - PubMed
-
- Auerbach C., Robson J. M., Carr J. G., 1947. The chemical production of mutations. Science 105: 243–247. - PubMed
-
- Bassett A., Liu J. L., 2014. CRISPR/Cas9 mediated genome engineering in Drosophila. Methods 69: 128–136. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

