Covalent Linkage of HIV-1 Trimers to Synthetic Liposomes Elicits Improved B Cell and Antibody Responses
- PMID: 28592540
- PMCID: PMC5533919
- DOI: 10.1128/JVI.00443-17
Covalent Linkage of HIV-1 Trimers to Synthetic Liposomes Elicits Improved B Cell and Antibody Responses
Abstract
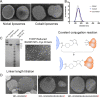
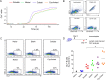
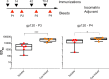
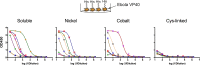
We have demonstrated that a liposomal array of well-ordered trimers enhances B cell activation, germinal center formation, and the elicitation of tier-2 autologous neutralizing antibodies. Previously, we coupled well-ordered cleavage-independent NFL trimers via their C-terminal polyhistidine tails to nickel lipids integrated into the lipid bilayer. Despite favorable in vivo effects, concern remained over the potentially longer-term in vivo instability of noncovalent linkage of the trimers to the liposomes. Accordingly, we tested both cobalt coupling and covalent linkage of the trimers to the liposomes by reengineering the polyhistidine tail to include a free cysteine on each protomer of model BG505 NFL trimers to allow covalent linkage. Both cobalt and cysteine coupling resulted in a high-density array of NFL trimers that was stable in both 20% mouse serum and 100 mM EDTA, whereas the nickel-conjugated trimers were not stable under these conditions. Binding analysis and calcium flux with anti-Env-specific B cells confirmed that the trimers maintained conformational integrity following coupling. Following immunization of mice, serologic analysis demonstrated that the covalently coupled trimers elicited Env-directed antibodies in a manner statistically significantly improved compared to soluble trimers and nickel-conjugated trimers. Importantly, the covalent coupling not only enhanced gp120-directed responses compared to soluble trimers, it also completely eliminated antibodies directed to the C-terminal His tag located at the "bottom" of the spike. In contrast, soluble and noncovalent formats efficiently elicited anti-His tag antibodies. These data indicate that covalent linkage of well-ordered trimers to liposomes in high-density array displays multiple advantages in vitro and in vivoIMPORTANCE Enveloped viruses typically encode a surface-bound glycoprotein that mediates viral entry into host cells and is a primary target for vaccine design. Liposomes with modified lipid head groups have a unique feature of capturing and displaying antigens on their surfaces, mimicking the native pathogens. Our first-generation nickel-based liposomes captured HIV-1 Env glycoprotein trimers via a noncovalent linkage with improved efficacy over soluble glycoprotein in activating germinal center B cells and eliciting tier-2 autologous neutralizing antibodies. In this study, we report the development of second-generation cobalt- and maleimide-based liposomes that have improved in vitro stability over nickel-based liposomes. In particular, the maleimide liposomes captured HIV-1 Env trimers via a more stable covalent bond, resulting in enhanced germinal center B cell responses that generated higher antibody titers than the soluble trimers and liposome-bearing trimers via noncovalent linkages. We further demonstrate that covalent coupling prevents release of the trimers prior to recognition by B cells and masks a nonneutralizing determinant located at the bottom of the trimer.
Keywords: HIV-1; antibody repertoire; human immunodeficiency virus; immunization; immunology; liposomes; nanoparticles; pathogens; vaccines.
Copyright © 2017 American Society for Microbiology.
Figures




References
-
- Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, Hanlon DJ. 2006. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology 117:78–88. doi: 10.1111/j.1365-2567.2005.02268.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

