Immune-Related Tumor Response Dynamics in Melanoma Patients Treated with Pembrolizumab: Identifying Markers for Clinical Outcome and Treatment Decisions
- PMID: 28592629
- PMCID: PMC5559305
- DOI: 10.1158/1078-0432.CCR-17-0114
Immune-Related Tumor Response Dynamics in Melanoma Patients Treated with Pembrolizumab: Identifying Markers for Clinical Outcome and Treatment Decisions
Abstract
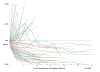
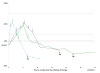
Purpose: Characterize tumor burden dynamics during PD-1 inhibitor therapy and investigate the association with overall survival (OS) in advanced melanoma.Experimental Design: The study included 107 advanced melanoma patients treated with pembrolizumab. Tumor burden dynamics were assessed on serial CT scans using irRECIST and were studied for the association with OS.Results: Among 107 patients, 96 patients had measurable tumor burden and 11 had nontarget lesions alone at baseline. In the 96 patients, maximal tumor shrinkage ranged from -100% to 567% (median, -18.5%). Overall response rate was 44% (42/96; 5 immune-related complete responses, 37 immune-related partial responses). Tumor burden remained <20% increase from baseline throughout therapy in 57 patients (55%). Using a 3-month landmark analysis, patients with <20% tumor burden increase from baseline had longer OS than patients with ≥20% increase (12-month OS rate: 82% vs. 53%). In extended Cox models, patients with <20% tumor burden increase during therapy had significantly reduced hazards of death [HR = 0.19; 95% confidence interval (CI), 0.08-0.43; P < 0.0001 univariate; HR = 0.18; 95% CI, 0.08-0.41; P < 0.0001, multivariable]. Four patients (4%) experienced pseudoprogression; 3 patients had target lesion increase with subsequent response, which was noted after confirmed immune-related progressive disease (irPD). One patient without measurable disease progressed with new lesion that subsequently regressed.Conclusions: Tumor burden increase of <20% from the baseline during pembrolizumab therapy was associated with longer OS, proposing a practical marker for treatment decision guides that needs to be prospectively validated. Pseudoprogressors may experience response after confirmed irPD, indicating a limitation of the current strategy for immune-related response evaluations. Evaluations of patients without measurable disease may require further attention. Clin Cancer Res; 23(16); 4671-9. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures




References
-
- Nishino M, Jagannathan JP, Krajewski KM, O'Regan K, Hatabu H, Shapiro G, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR American journal of roentgenology. 2012;198(4):737–45. doi: 10.2214/AJR.11.7483. - DOI - PMC - PubMed
-
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. - DOI - PubMed
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92(3):205–16. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

