The structural response of the cornea to changes in stromal hydration
- PMID: 28592658
- PMCID: PMC5493790
- DOI: 10.1098/rsif.2017.0062
The structural response of the cornea to changes in stromal hydration
Abstract
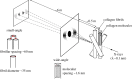
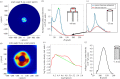
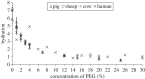
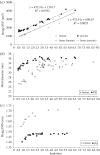
The primary aim of this study was to quantify the relationship between corneal structure and hydration in humans and pigs. X-ray scattering data were collected from human and porcine corneas equilibrated with polyethylene glycol (PEG) to varying levels of hydration, to obtain measurements of collagen fibril diameter, interfibrillar spacing (IFS) and intermolecular spacing. Both species showed a strong positive linear correlation between hydration and IFS2 and a nonlinear, bi-phasic relationship between hydration and fibril diameter, whereby fibril diameter increased up to approximately physiological hydration, H = 3.0, with little change thereafter. Above H = 3.0, porcine corneas exhibited a larger fibril diameter than human corneas (p < 0.001). Intermolecular spacing also varied with hydration in a bi-phasic manner but reached a maximum value at a lower hydration (H = 1.5) than fibril diameter. Human corneas displayed a higher intermolecular spacing than porcine corneas at all hydrations (p < 0.0001). Human and porcine corneas required a similar PEG concentration to reach physiological hydration, suggesting that the total fixed charge that gives rise to the swelling pressure is the same. The difference in their structural responses to hydration can be explained by variations in molecular cross-linking and intra/interfibrillar water partitioning.
Keywords: collagen; cornea; fixed charge; hydration; proteoglycans; swelling.
© 2017 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures



References
-
- Gyi TJ, Meek KM, Elliot GF. 1988. Collagen interfibrillar distances in the corneal stroma using synchrotron X-ray diffraction: a species study. Int. J. Biol. Macromol. 10, 265–269. (10.1016/0141-8130(88)90002-5) - DOI
-
- Komai Y, Ushiki T. 1991. The three-dimensional organisation of collagen fibrils in the human cornea and sclera. Invest. Ophthalmol. Vis. Sci. 32, 2244–2258. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
