Tetranychus urticae mites do not mount an induced immune response against bacteria
- PMID: 28592670
- PMCID: PMC5474072
- DOI: 10.1098/rspb.2017.0401
Tetranychus urticae mites do not mount an induced immune response against bacteria
Abstract
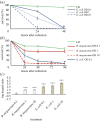
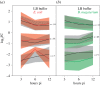
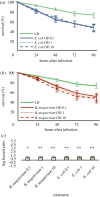
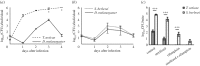
The genome of the spider mite Tetranychus urticae, a herbivore, is missing important elements of the canonical Drosophila immune pathways necessary to fight bacterial infections. However, it is not known whether spider mites can mount an immune response and survive bacterial infection. In other chelicerates, bacterial infection elicits a response mediated by immune effectors leading to the survival of infected organisms. In T. urticae, infection by either Escherichia coli or Bacillus megaterium did not elicit a response as assessed through genome-wide transcriptomic analysis. In line with this, spider mites died within days even upon injection with low doses of bacteria that are non-pathogenic to Drosophila Moreover, bacterial populations grew exponentially inside the infected spider mites. By contrast, Sancassania berlesei, a litter-dwelling mite, controlled bacterial proliferation and resisted infections with both Gram-negative and Gram-positive bacteria lethal to T. urticae This differential mortality between mite species was absent when mites were infected with heat-killed bacteria. Also, we found that spider mites harbour in their gut 1000-fold less bacteria than S. berlesei We show that T. urticae has lost the capacity to mount an induced immune response against bacteria, in contrast to other mites and chelicerates but similarly to the phloem feeding aphid Acyrthosiphon pisum Hence, our results reinforce the putative evolutionary link between ecological conditions regarding exposure to bacteria and the architecture of the immune response.
Keywords: Sancassania berlesei; Tetranychus urticae; host–parasite interactions; immunity; microbiota.
© 2017 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures
References
-
- Beckage NE. 2008. Insect immunology. San Diego, CA: Academic Press.
-
- Schmid-Hempel P. 2011. Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics. New York, NY: Oxford University Press.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
