Lower Affinity of Isradipine for L-Type Ca2+ Channels during Substantia Nigra Dopamine Neuron-Like Activity: Implications for Neuroprotection in Parkinson's Disease
- PMID: 28592699
- PMCID: PMC6596555
- DOI: 10.1523/JNEUROSCI.2946-16.2017
Lower Affinity of Isradipine for L-Type Ca2+ Channels during Substantia Nigra Dopamine Neuron-Like Activity: Implications for Neuroprotection in Parkinson's Disease
Abstract
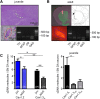
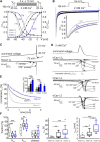
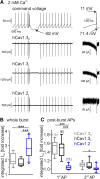
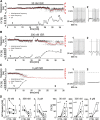
Ca2+-influx through L-type Ca2+-channels (LTCCs) is associated with activity-related stressful oscillations of Ca2+ levels within dopaminergic (DA) neurons in the substantia nigra (SN), which may contribute to their selective degeneration in Parkinson's disease (PD). LTCC blockers were neuroprotective in mouse neurotoxin models of PD, and isradipine is currently undergoing testing in a phase III clinical trial in early PD. We report no evidence for neuroprotection by in vivo pretreatment with therapeutically relevant isradipine plasma levels, or Cav1.3 LTCC deficiency in 6-OHDA-treated male mice. To explain this finding, we investigated the pharmacological properties of human LTCCs during SN DA-like and arterial smooth muscle (aSM)-like activity patterns using whole-cell patch-clamp recordings in HEK293 cells (Cav1.2 α1-subunit, long and short Cav1.3 α1-subunit splice variants; β3/α2δ1). During SN DA-like pacemaking, only Cav1.3 variants conducted Ca2+ current (ICa) at subthreshold potentials between action potentials. SN DA-like burst activity increased integrated ICa during (Cav1.2 plus Cav1.3) and after (Cav1.3) the burst. Isradipine inhibition was splice variant and isoform dependent, with a 5- to 11-fold lower sensitivity to Cav1.3 variants during SN DA-like pacemaking compared with Cav1.2 during aSM-like activity. Supratherapeutic isradipine concentrations reduced the pacemaker precision of adult mouse SN DA neurons but did not affect their somatic Ca2+ oscillations. Our data predict that Cav1.2 and Cav1.3 splice variants contribute differentially to Ca2+ load in SN DA neurons, with prominent Cav1.3-mediated ICa between action potentials and after bursts. The failure of therapeutically relevant isradipine levels to protect SN DA neurons can be explained by weaker state-dependent inhibition of SN DA LTCCs compared with aSM Cav1.2.SIGNIFICANCE STATEMENT The high vulnerability of dopamine (DA) neurons in the substantia nigra (SN) to neurodegenerative stressors causes Parkinson's disease (PD). Ca2+ influx through voltage-gated L-type Ca2+ channels (LTCCs), in particular Cav1.3, appears to contribute to this vulnerability, and the LTCC inhibitor isradipine is currently being tested as a neuroprotective agent for PD in a phase III clinical trial. However, in our study isradipine plasma concentrations approved for therapy were not neuroprotective in a PD mouse model. We provide an explanation for this observation by demonstrating that during SN DA-like neuronal activity LTCCs are less sensitive to isradipine than Cav1.2 LTCCs in resistance blood vessels (mediating dose-limiting vasodilating effects) and even at supratherapeutic concentrations isradipine fails to reduce somatic Ca2+ oscillations of SN DA neurons.
Keywords: L-type calcium channels; Parkinson's disease; calcium; isradipine; neuroprotection; pharmacology.
Copyright © 2017 the authors 0270-6474/17/376762-17$15.00/0.
Figures



References
-
- Allen GS, Ahn HS, Preziosi TJ, Battye R, Boone SC, Boone SC, Chou SN, Kelly DL, Weir BK, Crabbe RA, Lavik PJ, Rosenbloom SB, Dorsey FC, Ingram CR, Mellits DE, Bertsch LA, Boisvert DP, Hundley MB, Johnson RK, Strom JA, et al. (1983) Cerebral arterial spasm–a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med 308:619–624. 10.1056/NEJM198303173081103 - DOI - PubMed
-
- Amini B, Clark JW Jr, Canavier CC (1999) Calcium dynamics underlying pacemaker-like and burst firing oscillations in midbrain dopaminergic neurons: a computational study. J Neurophysiol 82:2249–2261. - PubMed
-
- Bock G, Gebhart M, Scharinger A, Jangsangthong W, Busquet P, Poggiani C, Sartori S, Mangoni ME, Sinnegger-Brauns MJ, Herzig S, Striessnig J, Koschak A (2011) Functional properties of a newly identified C-terminal splice variant of Cav1.3 L-type Ca2+ channels. J Biol Chem 286:42736–42748. 10.1074/jbc.M111.269951 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
