Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue
- PMID: 28592801
- PMCID: PMC5462798
- DOI: 10.1038/s41598-017-02660-w
Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue
Abstract
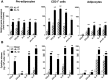
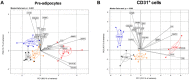
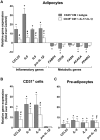
Adipose tissue contains a variety of immune cells, which vary in abundance and phenotype with obesity. The contribution of immune cell-derived factors to inflammatory, fibrotic and metabolic alterations in adipose tissue is not well established in human obesity. Human primary adipose tissue cells, including pre-adipocytes, endothelial cells and mature adipocytes, were used to investigate deregulation of cell- and pathway-specific gene profiles. Among factors known to alter adipose tissue biology, we focus on inflammatory (IL-1β and IL-17) and pro-fibrotic (TGF-β1) factors. rIL-1β and rIL-17 induced concordant pro-inflammatory transcriptional programs in pre-adipocytes and endothelial cells, with a markedly more potent effect of IL-1β than IL-17. None of these cytokines had significant effect on fibrogenesis-related gene expression, contrasting with rTGF-β1-induced up-regulation of extracellular matrix components and pro-fibrotic factors. In mature adipocytes, all three factors promoted down-regulation of genes functionally involved in lipid storage and release. IL-1β and IL-17 impacted adipocyte metabolic genes in relation with their respective pro-inflammatory capacity, while the effect of TGF-β1 occurred in face of an anti-inflammatory signature. These data revealed that IL-1β and IL-17 had virtually no effect on pro-fibrotic alterations but promote inflammation and metabolic dysfunction in human adipose tissue, with a prominent role for IL-1β.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

