SNX10 gene mutation leading to osteopetrosis with dysfunctional osteoclasts
- PMID: 28592808
- PMCID: PMC5462793
- DOI: 10.1038/s41598-017-02533-2
SNX10 gene mutation leading to osteopetrosis with dysfunctional osteoclasts
Abstract
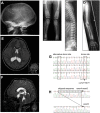
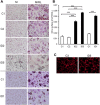
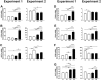
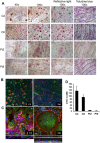
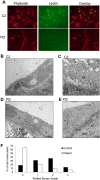
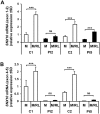
Autosomal recessive osteopetrosis (ARO) is a heterogeneous disorder, characterized by defective osteoclastic resorption of bone that results in increased bone density. We have studied nine individuals with an intermediate form of ARO, from the county of Västerbotten in Northern Sweden. All afflicted individuals had an onset in early infancy with optic atrophy, and in four patients anemia was present at diagnosis. Tonsillar herniation, foramen magnum stenosis, and severe osteomyelitis of the jaw were common clinical features. Whole exome sequencing, verified by Sanger sequencing, identified a splice site mutation c.212 + 1 G > T in the SNX10 gene encoding sorting nexin 10. Sequence analysis of the SNX10 transcript in patients revealed activation of a cryptic splice site in intron 4 resulting in a frame shift and a premature stop (p.S66Nfs * 15). Haplotype analysis showed that all cases originated from a single mutational event, and the age of the mutation was estimated to be approximately 950 years. Functional analysis of osteoclast progenitors isolated from peripheral blood of patients revealed that stimulation with receptor activator of nuclear factor kappa-B ligand (RANKL) resulted in a robust formation of large, multinucleated osteoclasts which generated sealing zones; however these osteoclasts exhibited defective ruffled borders and were unable to resorb bone in vitro.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

