PH-Dependent Enantioselectivity of D-amino Acid Oxidase in Aqueous Solution
- PMID: 28592826
- PMCID: PMC5462808
- DOI: 10.1038/s41598-017-03177-y
PH-Dependent Enantioselectivity of D-amino Acid Oxidase in Aqueous Solution
Abstract
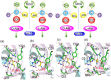
D-amino acid oxidases (DAAO) are stereospecific enzymes which are generally almost inactive towards L-enantiomer in neutral solution when L-, D-amino acids are supplied as substrates. In this paper, the D-amino acid oxidase can catalytic oxidize L-amino acids by modulating pH of aqueous solution. With L-Pro as substrate, the catalytic rate (k cat) and the affinity (K m) of DAAO were 6.71 s-1 and 33 mM at pH 8.0, respectively, suggesting that optimal pH condition enhanced the activity of DAAO towards L-Pro. Similar results were obtained when L-Ala (pH 9.8), L-Arg (pH 6.5), L-Phe (pH 9.0), L-Thr (pH 9.4), and L-Val (pH 8.5) were catalyzed by DAAO at various pH values. The racemization of the L-amino acids was not found by capillary electrophoresis analysis during oxidation, and quantification analysis of L-amino acids before and after catalytic reaction was performed, which confirmed that the modulation of enantioselectivity of DAAO resulted from the oxidation of L-amino acids rather than D-amino acids by changing pH. A mechanistic model was proposed to explain enhanced activity of DAAO towards L-amino acids under optimal pH condition.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Daniello A, et al. Biological role of D-amino acid oxidase and D-aspartate oxidase. Effects of D-amino acids. J Biol Chem. 1993;268:26941–26949. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

