High-precision spatial localization of mouse vocalizations during social interaction
- PMID: 28592832
- PMCID: PMC5462771
- DOI: 10.1038/s41598-017-02954-z
High-precision spatial localization of mouse vocalizations during social interaction
Abstract
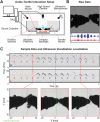
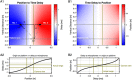
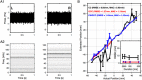
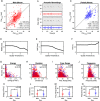
Mice display a wide repertoire of vocalizations that varies with age, sex, and context. Especially during courtship, mice emit ultrasonic vocalizations (USVs) of high complexity, whose detailed structure is poorly understood. As animals of both sexes vocalize, the study of social vocalizations requires attributing single USVs to individuals. The state-of-the-art in sound localization for USVs allows spatial localization at centimeter resolution, however, animals interact at closer ranges, involving tactile, snout-snout exploration. Hence, improved algorithms are required to reliably assign USVs. We develop multiple solutions to USV localization, and derive an analytical solution for arbitrary vertical microphone positions. The algorithms are compared on wideband acoustic noise and single mouse vocalizations, and applied to social interactions with optically tracked mouse positions. A novel, (frequency) envelope weighted generalised cross-correlation outperforms classical cross-correlation techniques. It achieves a median error of ~1.4 mm for noise and ~4-8.5 mm for vocalizations. Using this algorithms in combination with a level criterion, we can improve the assignment for interacting mice. We report significant differences in mean USV properties between CBA mice of different sexes during social interaction. Hence, the improved USV attribution to individuals lays the basis for a deeper understanding of social vocalizations, in particular sequences of USVs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
High-precision spatial analysis of mouse courtship vocalization behavior reveals sex and strain differences.Sci Rep. 2023 Mar 30;13(1):5219. doi: 10.1038/s41598-023-31554-3. Sci Rep. 2023. PMID: 36997591 Free PMC article.
-
Rodent ultrasonic vocal interaction resolved with millimeter precision using hybrid beamforming.Elife. 2023 Jul 26;12:e86126. doi: 10.7554/eLife.86126. Elife. 2023. PMID: 37493217 Free PMC article.
-
CBA/CaJ mouse ultrasonic vocalizations depend on prior social experience.PLoS One. 2018 Jun 6;13(6):e0197774. doi: 10.1371/journal.pone.0197774. eCollection 2018. PLoS One. 2018. PMID: 29874248 Free PMC article.
-
Determinants of the mouse ultrasonic vocal structure and repertoire.Neurosci Biobehav Rev. 2016 Jun;65:313-25. doi: 10.1016/j.neubiorev.2016.03.029. Epub 2016 Apr 7. Neurosci Biobehav Rev. 2016. PMID: 27060755 Review.
-
From Play to Aggression: High-Frequency 50-kHz Ultrasonic Vocalizations as Play and Appeasement Signals in Rats.Curr Top Behav Neurosci. 2017;30:91-108. doi: 10.1007/7854_2015_432. Curr Top Behav Neurosci. 2017. PMID: 26728173 Review.
Cited by
-
Rates of female mouse ultrasonic vocalizations are low and are not modulated by estrous state during interactions with muted males.Sci Rep. 2025 Feb 26;15(1):6841. doi: 10.1038/s41598-025-91479-x. Sci Rep. 2025. PMID: 40000725 Free PMC article.
-
Ultrasonic vocalization phenotypes in the Ts65Dn and Dp(16)1Yey mouse models of Down syndrome.Physiol Behav. 2023 Nov 1;271:114323. doi: 10.1016/j.physbeh.2023.114323. Epub 2023 Aug 11. Physiol Behav. 2023. PMID: 37573959 Free PMC article.
-
Rapid development of mature vocal patterns of ultrasonic calls in a fast-growing rodent, the yellow steppe lemming (Eolagurus luteus).PLoS One. 2020 Feb 11;15(2):e0228892. doi: 10.1371/journal.pone.0228892. eCollection 2020. PLoS One. 2020. PMID: 32045453 Free PMC article.
-
Biological and Acoustic Sex Differences in Rat Ultrasonic Vocalization.Brain Sci. 2021 Apr 4;11(4):459. doi: 10.3390/brainsci11040459. Brain Sci. 2021. PMID: 33916537 Free PMC article. Review.
-
Longitudinal analysis of ultrasonic vocalizations in mice from infancy to adolescence: Insights into the vocal repertoire of three wild-type strains in two different social contexts.PLoS One. 2019 Jul 31;14(7):e0220238. doi: 10.1371/journal.pone.0220238. eCollection 2019. PLoS One. 2019. PMID: 31365551 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

