Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL
- PMID: 28592889
- PMCID: PMC5770586
- DOI: 10.1038/leu.2017.175
Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL
Abstract
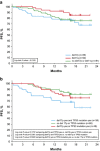
In the phase 3 RESONATE study, ibrutinib demonstrated superior progression-free survival (PFS), overall survival (OS) and overall response rate (ORR) compared with ofatumumab in relapsed/refractory CLL patients with high-risk prognostic factors. We report updated results from RESONATE in these traditionally chemotherapy resistant high-risk genomic subgroups at a median follow-up of 19 months. Mutations were detected by Foundation One Heme Panel. Baseline mutations in the ibrutinib arm included TP53 (51%), SF3B1 (31%), NOTCH1 (28%), ATM (19%) and BIRC3 (14%). Median PFS was not reached, with 74% of patients randomized to ibrutinib alive and progression-free at 24 months. The improved efficacy of ibrutinib vs ofatumumab continues in all prognostic subgroups including del17p and del11q. No significant difference within the ibrutinib arm was observed for PFS across most genomic subtypes, although a subset carrying both TP53 mutation and del17p had reduced PFS compared with patients with neither abnormality. Reduced PFS or OS was not evident in patients with only del17p. PFS was significantly better for ibrutinib-treated patients in second-line vs later lines of therapy. The robust clinical activity of ibrutinib continues to show ongoing efficacy and acceptable safety consistent with prior reports, independent of various known high-risk mutations.
Conflict of interest statement
JRB has received honoraria from Celgene, Gilead, Infinity, Genentech/Roche, Janssen, Pharmacyclics, Pfizer and Sub BioPharma, has served in an advisory role for Celgene, Gilead, Infinity, Genentech, Janssen, Pfizer and Pharmacyclics, and has been reimbursed for expenses by Gilead, Sun BioPharma, Pfizer and Janssen. PH has served as a consultant for Roche, Glaxo Smith Kline, Janssen, Gilead, and AbbVie, received honoraria from AbbVie, Gilead, Glaxo Smith Kline, Roche, Novartis, Pharmacyclics and Janssen, and research funding from Roche, Novartis, Glaxo Smith Kline, Janssen, Gilead, AbbVie, Celgene, Pharmacyclics. SOB has served as a consultant and received honoraria from Pharmacyclics and Janssen, and received research funding from Pharmacyclics. JCBarrientos has served as a consultant to Gilead, AbbVie, and Janssen, and received research funding from AbbVie and Gilead. NMR has served as a consultant for Celgene, Infinity, Gilead, and AbbVie. SEC has served in an advisory role for Janssen and Pharmacyclics, and has received research funding from AbbVie and Pharmacyclics. CT has received honoraria from Janssen, research funding from Janssen, and has served in an advisory role for Janssen. SPM has served as a consultant for and received honoraria from AbbVie, Gilead, Glaxo Smith Kline, Janssen, Roche, received research funding from AbbVie, Janssen, Roche, participated in speakers’ bureau for AbbVie, Gilead, Janssen, Roche. UJ has served as a consultant, and received honoraria and reimbursements from Janssen and Roche. PMB has served as a consultant for Pharmacyclics, AbbVie and received research funding from Pharmacyclics. RRF has received honoraria from Pharmacyclics, and has served in an advisory role and on Speakers’ Bureaus for Pharmacyclics. TJK has served as a consultant for AbbVie, Genentech, Gilead, and received research funding from AbbVie, Genentech, Pharmacyclics. FC has received research funding from Janssen, honoraria from Gilead, Janssen, Mundipharma and AbbVie, has consulted for Gilead, Janssen and AbbVie, and been reimbursed by Janssen, and Roche. PT has served as a consultant for Janssen. FCC has received honoraria and served as a consultant for Celgene, Janssen, Pharmacyclics. JD has received honoraria from and served as a consultant for Gilead, Novartis, Glaxo Smith Kline, Janssen, Roche, and received research funding from Infinity, Roche. MM has received honoraria from and served in a consultancy role for Roche, Gilead, and Janssen, and received honoraria from Novartis. SDV and CM have no relevant conflicts of interest to disclose. JMP has served as a consultant for Gilead and Pharmacyclics, and received research funding from Pharmacyclics, AbbVie, and TG Therapeutics. TM has received honoraria from Pharmacyclics, Gilead, and Alexion and has served as a consultant for Morphosys. JAB has served as a consultant for Janssen, Portola, has received research funding from Gilead, Pharmacyclics, and reimbursement from Janssen, Roche. DC is employed by Pharmacyclics and has stock/ownership in Gilead and AbbVie. JL, LG, BC, GC, EH, DFJ are employed by Pharmacyclics and have stock/ownership in AbbVie. JCByrd has no relevant conflict of interest to disclose.
Figures



References
-
- Catovsky D, Richards S, Matutes E, Oscier D, Dyer MJ, Bezares RF et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet 2007; 370: 230–239. - PubMed
-
- Flinn IW, Neuberg DS, Grever MR, Dewald GW, Bennett JM, Paietta EM et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol 2007; 25: 793–798. - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–1174. - PubMed
-
- Keating MJ, O'Brien S, Albitar M, Lerner S, Plunkett W, Giles F et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005; 23: 4079–4088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

